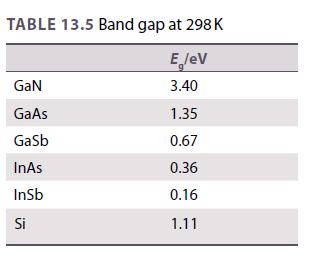
Use the data in Table 13.5 to estimate a band gap for the semiconductor GaP. Use this
Question:
Use the data in Table 13.5 to estimate a band gap for the semiconductor GaP. Use this to calculate the wavelength of light that is emitted when an electron drops from the conduction band to the valence band. Is this consistent with the observed green light of gallium phosphide LEDs?
Table 13.5.
Transcribed Image Text:
TABLE 13.5 Band gap at 298 K E lev 3.40 GaN GaAs Gasb InAs InSb Si 1.35 0.67 0.36 0.16 1.11
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
The band gap energy Eg of a semiconductor can be related to the wavelength of light it emits or abso...View the full answer

Answered By

Sayee Sreenivas G B
I have extensive tutoring experience, having worked as a private tutor for over three years. I have tutored students from different academic levels, including high school, undergraduate, and graduate levels. My tutoring experience has taught me to be patient, attentive to student needs, and effective in communicating difficult concepts in simple terms.
I have a strong background in statistics, probability theory, data analysis, and data visualization. I am proficient in using statistical software such as R, Python, and SPSS, which are commonly used in academic research and data analysis. Additionally, I have excellent communication and interpersonal skills, which enable me to establish rapport with students, understand their learning styles, and adapt my teaching approach to meet their needs.
I am passionate about teaching and helping students achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Light of wavelength 1.03 107 m is emitted when an electron in an excited level of a hydrogen atom undergoes a transition to the n = 1 level. What is the region of the spectrum of this light? What is...
-
Calculate the frequency (Hz) and wavelength (nm) of the emitted photon when an electron drops from the n = 4 to the n = 2 level in a hydrogen atom.
-
The maximum wavelength of light that a certain silicon photocell can detect is 1.11m. (a) What is the energy gap (in electron volts) between the valence and conduction bands for this photocell? (b)...
-
The net present value and internal rate of return desirability measures for two mutually exclusive investments being considered by Stockton Corporation to follow. Year NPV IRR R 161 14.60% S 138...
-
Panda Airlines Inc. has two divisions organized as profit centers, the Passenger Division and the Cargo Division. The following divisional income statements were prepared: The service department...
-
How effective are the interactions and interrelationships among the sub-systems? Give practical examples to support your view.
-
Which of the following is not a reason that firms need to innovate? a. Changing customer needs b. Preventing declining sales c. Diversifying risk d. Responding to short product life cycles e. All of...
-
You have just purchased a car and taken out a $50,000 loan. The loan has a five-year term with monthly payments and an APR of 6%. a. How much will you pay in interest, and how much will you pay in...
-
Explain in detail the static trade-off theory of capital structure. The reader should clearly understand each individual component involved in the trade-off. What does this theory have to say about...
-
Starting with B 10 H 14 and other reagents of your choice, give the equations for the synthesis of [Fe(nido-B 9 C 2 H 11 ) 2 ] 2 , and sketch the structure of this species.
-
Hydrolysis of 1 mol of a borohydride yields 15 mol of H 2 and 6 mol of B(OH) 3 . Identify the compound and suggest a structure.
-
Explain the importance of learning about operations management. LO.1
-
The composition of moist air is given on a molar basis to be 78 percent N2, 20 percent O2, and 2 percent water vapor. Determine the mass fractions of the constituents of air. Use the table containing...
-
1. Consider the LFSR with so = 1, 8 = 1, S2 = 1, 83 = 1, 84 = 0, and Sn Sn-2 Sn-3+ Sn-5. Find the next 15 terms in this LFSR. What is the period of this LFSR? 2. Suppose you learn that a Hill cipher...
-
Assume that you are thinking of a new acquisition campaign for SEDO, assuming that you want to convert people who are already engaged. Develop a big idea (in the communication) that you can use in...
-
You have a backend Amazon EC2 instance providing a web service to your web server instances. Your web servers are in a public subnet. You would like to block inbound requests from the internet to...
-
Consider the following task set. Task C T|D T1 20 50 40 T2 10 40 30 T3 5 20 15 a) Verify whether the task set is schedulable under DM using the processor utilization-based ap- proach. b) Verify...
-
Read the short article "Sweden shortens work Day to Six Hours" by "Alexandria Ingham" and apply at least 3 Management concepts to the idea in this article.
-
D Which of the following is considered part of the Controlling activity of managerial accounting? O Choosing to purchase raw materials from one supplier versus another O Choosing the allocation base...
-
Comment on each of the following statements. (a) Ln 2+ complexes are strong reducing agents. (b) In the solid state, Cp 2 YbF(THF) exists as a bridged dimer, while Cp 2 YbCl(THF) and Cp 2 YbBr(THF)...
-
Give a short account of aspects of the organometallic compounds formed by the lanthanoids and actinoids and highlight major differences between families of organometallic complexes of the d- and...
-
Discuss the following: (a) Many actinoid oxides are non-stoichiometric, but few lanthanoid oxides are. (b) The ion [NpO 6 ] 5 can be made in aqueous solution only if the solution is strongly...
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


