Use the data in Table 14.2 and the additional bond enthalpy data given here to calculate the
Question:
Use the data in Table 14.2 and the additional bond enthalpy data given here to calculate the enthalpy of hydrolysis of CCl4 and CBr4. Bond enthalpies/kJ mol−1: O–H = 463, H–Cl = 431, H–Br = 366.
Table 14.2.
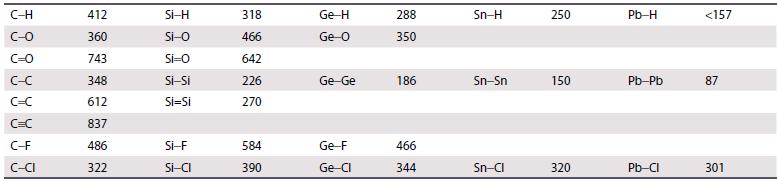
Transcribed Image Text:
C-H C-O C=0 C-C C=C C=C C-F C-CI 412 360 743 348 612 837 486 322 Si-H Si-O Si=O Si-Si Si=Si SI-F Si-CI 318 466 642 226 270 584 390 Ge-H Ge-0 Ge-Ge Ge-F Ge-Cl 288 350 186 466 344 Sn-H Sn-Sn Sn-Cl 250 150 320 Pb-H Pb-Pb Pb-Cl <157 87 301
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
To calculate the enthalpy of hydrolysis of CCl4 and CBr4 we need to consider the breaking of CX X Cl ...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Use Table 8.4 to estimate the enthalpy change for each of the following reactions: a. H2C == O (g) + HCl (g) H3C - O - Cl (g) b. H2O2 (g) + 2CO (g) H2 (g) + CO2 (g) (c). 3H2C == CH2 (g) C6H12 (g)...
-
Use the data in Table 2.7 to calculate the standard enthalpy of the reaction 2 H 2 (g) + O 2 (g) 2 H 2 O(g). The experimental value is 484 kJ. Account for the difference between the estimated and...
-
The "plastic" explosive C-4, often used in action movies, contains the molecule cyclotrimethylenetrinitramine, which is often called RDX (for Royal Demolition eXplosive):...
-
Project P costs $15,000 and is expected to produce benefits (cash flows) of $4,500 per year for five years. Project Q costs $37,500 and is expected to produce cash flows of $11,100 per year for five...
-
The Customer Service Department of Bragg Inc. asked the Publications Department to prepare a brochure for its training program. The Publications Department delivered the brochures and charged the...
-
Find a parametric representation for the surface. The part of the cylinder y 2 + z 2 = 16 that lies between the planes x = 0 and x = 5
-
5. For the example of the call option in Section 2, verify with a numerical example that the value of the call is increasing in the spread of the prices around the mean of $40.
-
The Crescent Drilling Company owns the drilling rights to several tracts of land on which natural gas has been found. The amount of gas on some of the tracts is somewhat marginal, and the company is...
-
Lusail Company builds office buildings. The office buildings are constructed under contract with reputable buyers. During 2016, Lusail Company began construction of an office building for AlKhor...
-
Discuss the solid-state chemistry of silicon in silicates with reference to how the various structures can be built up from SiO 4 tetrahedra linked into polymeric anions, chains, rings, sheets, and...
-
The lightest p-block elements often display different physical and chemical properties from the heavier members. Discuss the similarities and differences by comparison of: (a) The structures and...
-
Samuel Company has the following accounts in its shareholders equity section at the beginning of the current year: Common stock ($ 1 par, 1,000,000 shares authorized, 600,000 shares issued and...
-
How have your organizations performed relative to improving healthcare quality and meeting the required standards (Medicare metrics) for value-based purchasing initiatives?
-
/ Precalculus Algebra Problem. 1: Consider the function f(x)=-5x5 + +-4. How many terms in f(x) are not monomials? Problem. 2: Consider the function f(x)=-3x-4x - 3x + 12. How many terms in f(x) are...
-
D 0
-
What NaCl concentration results when 279 mL of a 0.680 M NaCl solution is mixed with 462 mL of a 0.450 M NaCl solution? concentration: M
-
Use JavaFX's shape's classes from javafx.scene.shape package to complete the following questions (Hint: CANNOT use any Gaphics or Graphics2D classes from java.awt packages): DO not write the whole...
-
As part of a feint attack, your firm (firm A) announces that in the next year, it intends to enter country X where the competitor (firm B) is very strong. Your firm's real intention is to march into...
-
If a test has high reliability. O the test measures what the authors of the test claim it measures O people who take the same test twice get approximately the same scores both times O scores on the...
-
Why is metal substitution used to investigate the metal binding site in carbonic anhydrase? Discuss the type of information that might be forthcoming from such a study.
-
Discuss the role of Zn 2+ as an example of a Lewis acid at work in a biological system.
-
(a) What is the function of cytochrome c oxidase? (b) Describe the four active metal-containing sites in cytochrome c oxidase and the proposed way in which they work together to fulfil the role of...
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


