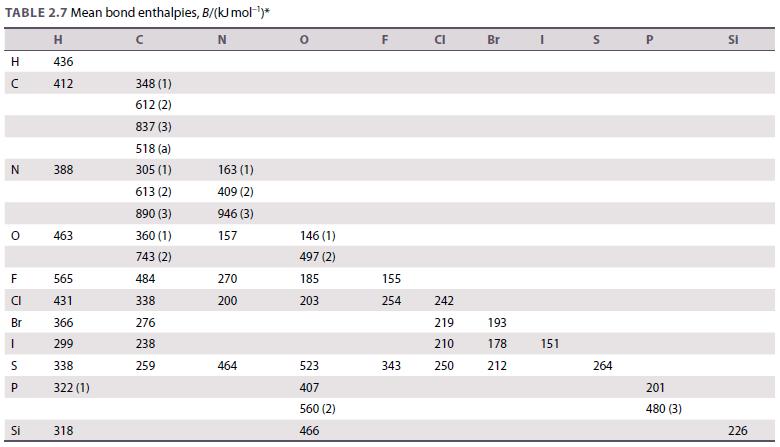
Use the data in Table 2.7 to calculate the standard enthalpy of the reaction 2 H 2
Question:
Use the data in Table 2.7 to calculate the standard enthalpy of the reaction 2 H2(g) + O2(g) → 2 H2O(g). The experimental value is −484 kJ. Account for the difference between the estimated and experimental values.
Table 2.7.
Transcribed Image Text:
TABLE 2.7 Mean bond enthalpies, B/(kJ mol-¹)* H N 436 412 H с N O F CI Br LL 1 S P Si 388 463 565 431 366 299 338 322 (1) 318 348 (1) 612 (2) 837 (3) 518 (a) 305 (1) 613 (2) 890 (3) 360 (1) 743 (2) 484 338 276 238 259 163 (1) 409 (2) 946 (3) 157 270 200 464 146 (1) 497 (2) 185 203 523 407 560 (2) 466 F 155 254 343 CI 242 219 210 250 Br 193 178 212 I 151 S 264 P 201 480 (3) SI 226
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
To calculate the standard enthalpy of the reaction 2 H2g O2g 2 H2Og using the data in Table 27 we will use Hesss Law which states that the overall ent...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
In the following exercises, you will use the data in the Solmaris Condominium Group database shown in Figures 1-21 through 1-25. (If you use a computer to complete these exercises, use a copy of the...
-
The personnel director of a firm has developed two tests to help determine whether potential employees would perform successfully in a particular position. To help estimate the usefulness of the...
-
The combustion of 0.4196 g of a hydrocarbon releases 17.55 kJ of heat. The masses of the products are CO2 = 1.419 g and H2O = 0.290 g. (a) What is the empirical formula of the compound? (b) If the...
-
9:49 X Bank Reconciliation Assignment 1 PDF 150 KB Assignment #1 - Bank Reconciliation This assignment has 33 total marks. This assignment is worth 5% of your final grade. This assignment is due...
-
Bond P is a premium bond with a 10 percent coupon. Bond D is a 4 percent coupon bond currently selling at a discount. Both bonds make annual payments, have a YTM of 7 percent, and have eight years to...
-
In Exercises 16, consider a Markov chain with state space {1,2,...........,n} and the given transition matrix. Find the communication classes for each Markov chain, and state whether the Markov chain...
-
2. Identify and discuss real examples of companies with a competitive advantage based on customer lock-in as opposed to product innovation. Which type of competitive advantage do you expect to...
-
The following information was taken from the accounts of Healthy Eats, a delicatessen, at December 31, 2013. The accounts are listed in alphabetical order, and each has a normal balance. Accounts...
-
Bot Corporation has 10,000 shares of 8%, $100 par value, cumulative preferred stock outstanding at December 31, 2022. No dividends were declared in 2020 or 2021
-
Use the Ketelaar triangle in Fig. 2.28 and the electronegativity values in Table 1.7 to predict what type of bonding is likely to dominate in (a) BCl 3 , (b) KCl, (c) BeO. Figure 2.28. Table 1.7. 3 2...
-
Use the covalent radii in Table 2.6 to calculate the bond lengths in (a) CCl 4 (177 pm), (b) SiCl 4 (201 pm), (c) GeCl 4 (210 pm). (The values in parentheses are experimental bond lengths and are...
-
Determine the sum of the first n terms of the geometric sequence for the values of a 1 and r. n = 6, a 1 = 1, r = -2
-
How has face book influenced political candidate's electoral success? What is the relationship between social media technology called face book and electoral success?
-
1. Discuss the international strategies that organizations can pursue 2. Identify and compare the various modes of of foreign market entry 3. Analyse the industry market 4. Evaluate relevant macro...
-
A bulk carrier was underway. The vessel was in ballast and hold washing was scheduled in preparation for taking the next cargo. An officer, bosun, and another deck crew conducted a risk assessment...
-
8. Neutrino radiation was observed over a certain period and the number of hours in which 0, 1, 2,... signals were received was recorded. 0 1 Number of Number of Hours with Signals per Hour This...
-
What are some advantages and disadvantages of centralization and decentralization. References: Altamimi, H., Liu, Q., & Jimenez, B. (2023). Not Too Much, Not Too Little: Centralization,...
-
When may reference to other agreements be made in a negotiable instrument without destroying its negotiability?
-
What services are provided by the provincial and territorial governments?
-
The industrial manufacture of NH 3 from N 2 and H 2 is carried out on a huge scale using heterogeneous catalysis, i.e. the reaction between gaseous N 2 and H 2 is carried out over a solid catalyst....
-
Provide explanations for the following observations. (a) In moist air, corrosion of iron is spontaneous. However, under anaerobic (O 2 free), wet conditions, corrosion of iron is only marginally...
-
A plumber directly connects a galvanized steel pipe to a copper pipe in a system that carries running water. Suggest what will happen over a period of time.
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


