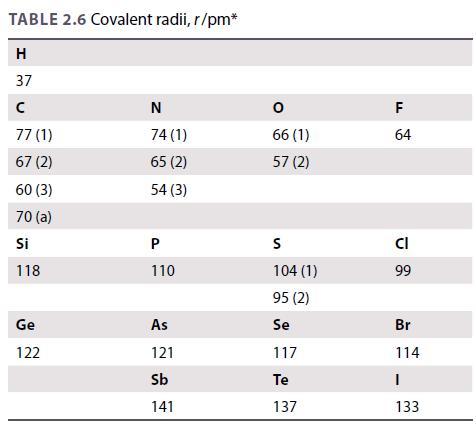
Use the covalent radii in Table 2.6 to calculate the bond lengths in (a) CCl 4 (177
Question:
Use the covalent radii in Table 2.6 to calculate the bond lengths in
(a) CCl4 (177 pm),
(b) SiCl4 (201 pm),
(c) GeCl4 (210 pm).
(The values in parentheses are experimental bond lengths and are included for comparison.)
Table 2.6.
Transcribed Image Text:
TABLE 2.6 Covalent radii, r/pm* H 37 с 77 (1) 67 (2) 60 (3) 70 (a) Si 118 Ge 122 N 74 (1) 65 (2) 54 (3) P 110 As 121 Sb 141 0 66 (1) 57 (2) S 104 (1) 95 (2) Se 117 Te 137 F 64 CI 99 Br 114 I 133
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To calculate the bond lengths in the given molecules CCl4 SiCl4 and GeCl4 using the covalent radii i...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Calculate the CH and CCl bond lengths in chloroform, CHCl3, using values for the covalent radii from Table 9.4. How do these values compare with the experimental values: CH, 107 pm; CCl, 177 pm?...
-
Calculate the CCl and CC bond lengths in ethyl chloride, C 2 H 5 Cl, using values for the covalent radii from Table 9.4. How do these values compare with the experimental values: CCl, 177; CC, 155...
-
Calculate the bond length for each of the following single bonds, using covalent radii (Table 9.4): a. CH b. SCl c. BrCl d. SiO TABLE 9.4 Single-Bond Covalent Radii for Nonmetallic Elements (in pm)...
-
Number of toys produced 60,000 120,000 150,000 O $0.20 What is the materials cost per unit of output? O $0.30 Starfun Toys, Inc. Cost of Materials O $0.70 O $0.50 Total cost of materials $18,000...
-
You want to have $2 million in real dollars in an account when you retire in 40 years. The nominal return on your investment is 10 percent and the inflation rate is 3.8 percent. What real amount must...
-
To practice the methods of this section, do not use an eigenvector routine from your matrix program. Instead, use the program to find the eigenvalues, and, for each eigenvalue , find an orthonormal...
-
3. Why do companies operating within the pharmaceutical and biotechnology industries typically sustain higher ROICs than firms in the hardware and equipment industries?
-
In a certain chemical process three bottles of a standard fluid are emptied into a larger container. A study of the individual bottles shows that the mean value of the contents is 15 ounces and the...
-
I need help on those I made a mistake! Thank you so much! Keep-Or-Drop Decision, Alternatives, Relevant Costs Reshier Company makes three types of rug shampooers. Model 1 is the basic model rented...
-
Use the data in Table 2.7 to calculate the standard enthalpy of the reaction 2 H 2 (g) + O 2 (g) 2 H 2 O(g). The experimental value is 484 kJ. Account for the difference between the estimated and...
-
What shapes would you expect for the species (a) ClF 3 , (b) lCl 4 , (c) I 3 ?
-
Which set of values, cultural diversity models, and multicultural paradigm management systems have been viewed? Describe philosophical and practical foundations of each model.
-
Over the past 40 years, union membership has declined, and it continues to do so. Instead, many companies are turning to alternative dispute resolution. We know one of the best union avoidance...
-
Article "A Leader's Journey" by Pamela Kruger Photographs by Nigel Dickson. For this discussion, let's try and unpack the key factors that led to his transformation. 1. What are your key takeaways...
-
Describe the collaborative roles of the team leader and the team coach in helping a group of people come together to form a team. Recommend strategies for Alex as team leader to use in helping to...
-
a. Complete the table with all marginal totals and cell counts. b. Calculate the following probabilities. i. For a male to be a Republican. ii. For a voter to be female. iii. For a voter to be either...
-
1. Will the Coronavirus Pandemic Make Working from Home the New Normal?" Address the following below. Define the problem described in this case. What are the management, organization, and technology...
-
What are some of the practical limitations concerning the writing evidencing a negotiable instrument and the substance on which it is placed?
-
An example of prescriptive analytics is when an action is recommended based on previously observed actions. For example, an analysis might help determine procedures to follow when new accounts are...
-
Consider the following two scenarios: (i) Aluminium rivets used to connect two steel plates, (ii) Steel rivets used to connect two Aluminium plates. Discuss whether these choices would be sensible.
-
The commercial purification of copper metal is carried out in electrolytic cells. The anode is composed of impure (blister) copper, and the electrolyte is a mixture of aqueous CuSO 4 and H 2 SO 4 ....
-
In each of the following reactions, relate starting materials and products by the processes of reduction, oxidation, disproportionation or no redox change. In some reactions, more than one process is...
-
Show that the convexity for a zero coupon bond with m payments per year is (m) n(n + -)(1+ m m
-
Abdul Canarte , a Central Bank economist, noticed that the total group purchasing basket of goods (CPI) has gone from $149,740.00 to $344,460.00 in 8 years. With monthly compounding, what is the...
-
ABC Corporation expects sales next year to be $50,000,000. Inventory and accounts receivable (combined) will increase $8,000,000 to accommodate this sales level. The company has a profit margin of 6...

Study smarter with the SolutionInn App


