Use the Latimer diagrams to determine which species of N and P disproportionate in acid conditions. Acidic
Question:
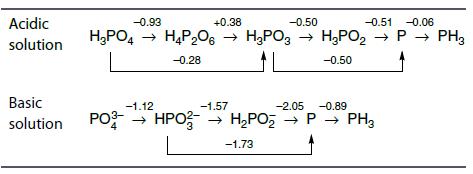
Use the Latimer diagrams to determine which species of N and P disproportionate in acid conditions.
Transcribed Image Text:
Acidic solution -0.93 +0.38 -0.50 -0.51 -0.06 H₂PO4 → H4P₂06 → H3PO3 → H₂PO₂ → P→ PH3 -0.28 -0.50 Basic -1.57 -2.05 -0.89 solution PO3- HPO → H₂PO₂ → H₂PO₂ → P→ PH3 -1.73 -1.12
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
In the Latimer diagrams provided for nitrogen N and phosphorus P species the species that disproport...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Figure is a digraph representing a food web in a small ecosystem. A directed edge from a to b indicates that a has b as a source of food. Construct the adjacency matrix A for this digraph and use it...
-
Find the mean, variance, and standard deviation for each of the values of n and p when the conditions for the binomial distribution are met. a. n = 100, p = 0.75 b. n = 300, p = 0.3 c. n = 20, p =...
-
Find the mean, variance, and standard deviation for each of the values of n and p when the conditions for the binomial distributions are met. a. n = 1000, p = 0.1 b. n = 500, p = 0.25 c. n = 50, p =...
-
Suppose that the vice president of marketing asks you to write a program to create labels for a onetime advertising promotion. As IT manager, you know that the labels can be prepared more efficiently...
-
Indio Palms College wishes to monitor the efficiency and quality of its course registration process. a. List three input and three output measures for this process. b. Why would Indio Palms College...
-
A bright yellow sodium emission line has a wavelength of 587.561 8 nm. Determine the difference between the atoms two energy levels defining the transition. Give your answer in eV to four significant...
-
15. Explain why the VIX formula in equation (29) overestimates implied volatility if options are American.
-
Alpha Products, Inc., is having a problem trying to control inventory. There is insufi cient time to devote to all its items equally. Here is a sample of some items stocked, along with the annual...
-
Vulcan Plyover's Operating Data For the Month Ended July 31 Actual Results 55 Flexible Planning Budget Budget 55 53 Flights (9) $ 16,100 $ 18,700 518,020 Revenue ($340.009) Expenses Wages and...
-
Write the balanced chemical equation corresponding to the standard enthalpy of formation of P 4 O 10 (s). Specify the structure, physical state (s, l, or g), and allotrope of the reactants. Does...
-
Starting with NH 3 (g) and other reagents of your choice, give the chemical equations and conditions for the synthesis of (a) HNO 3 , (b) NO 2 , (c) NH 2 OH, (d) N 3 .
-
Evaluate 13.92 5.31 and 13.92 5.31. Assuming that these values are correctly rounded numbers, calculate error bounds for each answer and write them as correctly rounded numbers which have the...
-
7. Chicago Corp. obtained the following information from the Raw Materials Inventory account and purchasing records for the first quarter of the current year: Beginning Raw Materials Ending Raw...
-
Suppose that i t =6% (n=1), and that future short term interest rates (n=1) for the next 3 years (starting next year) are expected to be: 4%, 2%, 2%. Suppose that the liquidity premium is zero for...
-
Mechanical Vibrations HW Use the modal analysis and numerical integration to compute and plot the time response of the system, which has the equations of motion [8 0 01 (1) 48 -12 01(x1 0 0 8 02-12...
-
Submit excel file with graph and exchange rate analysis. FOREIGN EXCHANGE RATESTHE YEN FOR DOLLARS. The Federal Reserve System Web site, www.federalreserve.gov/releases/H10/hist , provides historical...
-
Part 1: There are many types of communication styles used in the workplace. Choose what you think is your leadership style: north, south, east, or west. Click The Leadership Compass Self-Assessment...
-
1. Does increased World Trade mean increased risk? 2. Comment on the assumption, "If people are serious about doing business with you, they will speak English."
-
The following selected information was taken from Sun Valley Citys general fund statement of revenues, expenditures, and changes in fund balance for the year ended December 31, 2019: Revenues:...
-
(a) The following complexes each possess one of the structures listed in Table 19.4. Use the point group to deduce each structure: [ZnCl 4 ] 2 (T d ); [AgCl 3 ] 2 (D 3h ); [ZrF 7 ] 3 (C 2v ); [ReH 9...
-
Interactions between DNA and metal complexes are the basis for the use of square planar platinum(II)-containing anti-cancer drugs. (a) Explain how the interaction of right-handed DNA with chiral...
-
(a) In the solid state, Fe(CO) 5 possesses a trigonal bipyramidal structure. How many carbon environments are there? (b) Explain why only one signal is observed in the 13 C NMR spectrum of solutions...
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


