Using data in Table 7.5, determine the relative solubilities of Ca(OH) 2 and Mg(OH) 2 and explain
Question:
Using data in Table 7.5, determine the relative solubilities of Ca(OH)2 and Mg(OH)2 and explain the relevance of your answer to the extraction of magnesium from seawater.
Table 7.5.
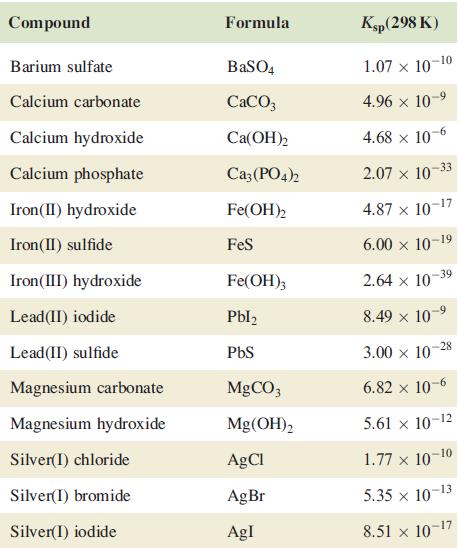

Transcribed Image Text:
Compound Barium sulfate Calcium carbonate Calcium hydroxide Calcium phosphate Iron (II) hydroxide Iron (II) sulfide Iron(III) hydroxide Lead (II) iodide Lead (II) sulfide Magnesium carbonate Magnesium hydroxide Silver(I) chloride Silver(1) bromide Silver(1) iodide Formula BaSO4 CaCO3 Ca(OH)2 Ca3(PO4)2 Fe(OH)2 FeS Fe(OH)3 Pbl₂ PbS MgCO3 Mg(OH)2 AgCl AgBr AgI Ksp (298 K) 1.07 x 10-10 4.96 × 10-⁹ 4.68 x 10-6 2.07 x 10 4.87 x 10-17 6.00 × 10-19 2.64 x 10-39 8.49 × 10-9 3.00 x 10 6.82 x 10-6 5.61 × 10-12 1.77 x 10-10 5.35 × 10-13 8.51 x 10-1 -17 -33 -28
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Based on the data provided in Table 75 we can determine the relative solubilities of CaOH2 and MgOH2 by comparing their solubility product constants K...View the full answer

Answered By

Surendar Kumaradevan
I have worked with both teachers and students to offer specialized help with everything from grammar and vocabulary to challenging problem-solving in a range of academic disciplines. For each student's specific needs, I can offer explanations, examples, and practice tasks that will help them better understand complex ideas and develop their skills.
I employ a range of techniques and resources in my engaged, interesting tutoring sessions to keep students motivated and on task. I have the tools necessary to offer students the support and direction they require in order to achieve, whether they need assistance with their homework, test preparation, or simply want to hone their skills in a particular subject area.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using data in Table 4.2 and assuming t/2R=0.07, compute the average circumferential stress in the ascending aorta, thoracic aorta, and femoral artery. Table 4.2: Values of the "pressure-strain", Ep,...
-
The accompanying stem and leaf plots were generated using MINITAB for the variables named "Popular Vote" and "Percent Vote." For Information: Refer to Exercises 1.51 and 1.52. Exercises 1.51 and 1.52...
-
The data listed in the following table relate to a study by Reiter and others (1981) concerning the effects of injecting triethyl-tin (TET) into rats once at age 5 days. The animals were injected...
-
When something burns, a. it combines with phlogiston b. it gives off phlogiston c. it combines with oxygen d. it gives off oxygen
-
Elite Classic Clothes is a retailer that sells to professional women in the northeast. The firm leases space for stores in upscale shopping centers, and the organizational structure consists of...
-
Use a Riemann sum with n = 4 and left endpoints to estimate the area under the graph in Fig. 1 for 0 x 2. y 20 10 0 (.5, 14) Figure 1 .5 (1, 10) 1 (1.5, 6) 1.5 (2, 4) 2 X
-
The following was the expenditure on a contract for Rs 6,00,000 commenced in January, 1997: Material Rs 1,20,000; Wages Rs 1,64,400; Plant Rs 20,000; Business Charges Rs 8,600. Cash received on...
-
Production workers for Kennedy Manufacturing Company provided 300 hours of labor in January and 600 hours in February. Kennedy expects to use 5,000 hours of labor during the year. The rental fee for...
-
Financial Transaction for the Month of September Details Below: Sept 1. Ivanna invested $200,000 Cash and a Building worth $500,000 to start her Legal Firm Sept. 2 Purchased a Company Vehicle for...
-
(a) Explain how face-sharing between M 6 O octahedra leads to compounds with stoichiometries of M 9 O 2 for M = Rb, and M 11 O 3 for M = Cs. (b) The suboxide Cs 7 O contains Cs 11 O 3 clusters....
-
Suggest explanations for the following observations. (a) Although Na 2 O 2 is described as being colourless, samples of Na 2 O 2 often appear to be very pale yellow. (b) NaO 2 is paramagnetic.
-
Explain how different types of definitions are based upon intension and extension in logic? (Use examples)
-
United States Historyassassination of Martin Luther King, Jr.
-
United States History-Burr-Hamilton duel duel, Weehawken, New Jersey, United States [1804]
-
United States HistoryBattle of Gettysburg American Civil War [1863] When and where was the Battle of Gettysburg fought?
-
United States History - United States presidential election of 1968 United States government
-
Salem witch trials American history What caused the Salem witch trials? How many people were killed during the Salem witch trials?
-
Nellie is evaluating a potential bond purchase that the seller purchased 12 years ago for $4,000. The bond matures 8 years from today. It has a face value of $10,000, pays quarterly coupons with a...
-
Match each of the key terms with the definition that best fits it. _______________ A record of the sequence of data entries and the date of those entries. Here are the key terms from the chapter. The...
-
The 31 P-MAS-NMR spectrum of solid PCl 5 shows two resonances, one of which has a chemical shift similar to that found for 31 P in the salt CsPCl 6 . Explain.
-
Explain why the 13 C-NMR spectrum of [Fe 3 (CO) 12 ] shows only a single peak at 212.5 ppm at room temperature while the IR spectrum shows the presence of both terminal and bridging carbonyl groups....
-
Explain the observation that the 19 F-NMR spectrum of the [XeF 5 ] anion consists of a central peak symmetrically flanked by two peaks, each of which is roughly one-sixth of the intensity of the...
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...

Study smarter with the SolutionInn App


