Using electronegativity values of 1.57 for Be and 0.79 for Cs and a Ketelaar triangle (Fig. 2.28),
Question:
Using electronegativity values of 1.57 for Be and 0.79 for Cs and a Ketelaar triangle (Fig. 2.28), predict what type of compound might form between these elements.
Figure 2.28.
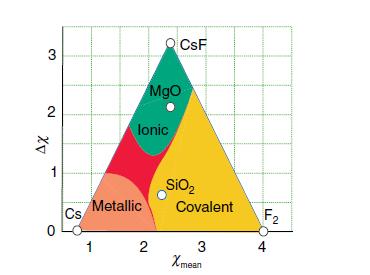
Transcribed Image Text:
3 2 ΔΧ 1 0 Cs Metallic 1 lonic MgO CsF 2 SiO₂ Covalent 3 Xmean F₂ 4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
To predict the type of compound that might form between beryllium Be and cesium Cs we can use the el...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Use the Ketelaar triangle in Fig. 2.28 and the electronegativity values in Table 1.7 to predict what type of bonding is likely to dominate in (a) BCl 3 , (b) KCl, (c) BeO. Figure 2.28. Table 1.7. 3 2...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
A five-year follow-up study was carried out in a certain metropolitan area to assess the relationship of diet and weight to the incidence of stomach cancer. Data were obtained on n = 2,000 subjects....
-
Consider two farmers, A and B, produce farm products and sell in the same market. Assume that the supply of the two farmers products are the same but the demand for Farmer Bs product is relatively...
-
At a professional conference just a few days ago, Darcy Kramer, the president of Luard Corporation, learned how the concept of controllability relates to performance evaluation. In preparing to put...
-
Cooling water for a chemical plant must be pumped from a river 2,500 ft from the plant site. Preliminary design calls for a flow of 600 gal/min and 6-in. steel pipe. Calculate the pressure drop and...
-
The 2004 election. George Bush was reelected president in 2004 with 51.0% of the popular vote. His Democratic opponent, John Kerry, received 48.1% of the vote, with minor candidates taking the...
-
Suppose you have the following historical returns for the stock market and for another company, P. Q. Unlimited. Explain how to calculate beta, and use the historical stock returns to calculate the...
-
Kiona Company set up a petty cash fund for payments of small amounts. The following transactions involving the petty cash fund occurred in May (the last month of the company's fiscal year) Hay 1...
-
Which salts of francium would be the least soluble? How could francium be precipitated and isolated from solution that also contains sodium ions?
-
The molecular geometries of crown ether derivatives play an important role in capturing and transporting alkali metal ions. K. Okano and co-workers (see K. Okano, H. Tsukube, and K. Hori,...
-
Sunglass Heaven, Inc., is launching a new store in a shopping mall in Houston. The annual revenue of the store depends on the weather conditions in the summer in Houston. The annual revenue will be...
-
Current Attempt in Progress The adjusted trial balance of Anthony Co. for the year ending December 31, 2025, contains the following. Anthony Co. Adjusted Trial Balance December 31, 2025 Debit Credit...
-
The coefficient of performance (COP) for a heat pump used as a heater (of a house, for example) is defined as 0=-QH/W, the ratio of the total heat flow -QH into the hot place (the house) to the work...
-
6 . A cylindrical furnace is operating at a temperature of 1 2 0 0 K and is emitting radiation uniformly in all directions. The inside diameter of the furnace is 2 m and the length of the furnace is...
-
How trade creates value ( Chapter 2 ) Max Daily Production Steaks Shrimp ( lbs . ) Fry Daddy 5 0 2 0 0 Grill Master 4 0 8 0 Refer to the above production data table for Fry Daddy and Grill Master....
-
Compounds A and B have the following vapor pressures: 150 o F: PA=600mmHg PB=500mmHg 200 o F: PA=1000mmHg PB=950mmHg Assume that these compounds form ideal solution, calculate the...
-
1. Understand human resource management and explain how managers develop and implement a human resource plan. 2. Understand why teams may be effective or ineffective and identify the skills needed by...
-
Write an SQL statement to display all data on products having a QuantityOnHand greater than 0.
-
Does VB theory indicate that the diatomic molecule He 2 is a viable species? Rationalize your answer.
-
(a) Draw resonance structures for CO, choosing only those that you think contribute significantly to the bonding. (b) Figure 2.15a shows an MO diagram for CO. Two MOs are illustrated by schematic...
-
Using VB theory and the Lewis structure model, determine the bond order in (a) H 2 , (b) Na 2 , (c) S 2 , (d) N 2 (e) Cl 2 . Is there any ambiguity with finding the bond orders by this method?
-
thumbs up if correct A stock paying no dividends is priced at $154. Over the next 3-months you expect the stock torpeither be up 10% or down 10%. The risk-free rate is 1% per annum compounded...
-
Question 17 2 pts Activities between affiliated entities, such as a company and its management, must be disclosed in the financial statements of a corporation as O significant relationships O segment...
-
Marchetti Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,000 cases of wine at a price of 200 euros per case. The total purchase price is 200,000...

Study smarter with the SolutionInn App


