What is the denticity of the following molecules? Which could act as bridging ligands? Which could act
Question:
What is the denticity of the following molecules? Which could act as bridging ligands? Which could act as chelating ligands?
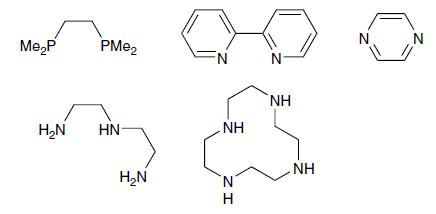
Transcribed Image Text:
Me₂P PMe₂ H₂N HN H₂N FN .ΝΗ ZI N H N NH NH N
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
To determine the denticity of the molecules and identify bridging and chelating ligands lets first understand what denticity bridging and chelating ligands mean Denticity Denticity refers to the numbe...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
How many bonds could each of the following chelating ligands form with a metal ion? a. Acetylacetone b. Diethylene triamine c. Salen d. Porphine CH2-C-CH NH2-CH-CH-NH-CH2-CH2-NH2 OH HO NH N N HN
-
Chelating ligands often form more stable complex ions than the corresponding monodentare ligands form with the same donor atoms. For example, where en is ethylenediamine and penten is The increased...
-
The carbonate ion (CO32-) can act as either a monodentate or a bidentate ligand. Draw a picture of CO32- coordinating to a metal ion as a bidentate and as a monodentate ligand. The carbonate ion can...
-
P Corporation acquired an 80% interest in S Corporation two years ago at an implied value equal to the book value of S. On January 2, 2017, S sold equipment with a five-year remaining life to P for a...
-
The following represents the potential outcomes of your first salary negotiation after graduation: Assuming this is a sequential move game with the employer moving first, indicate the most likely...
-
What is the difference between substitutes and complements? Indicate two goods that are substitutes for each other. Indicate two goods that are complements.
-
Density curves. (a) Sketch a density curve that is symmetric but has a shape different from that of the Normal curves. (b) Sketch a density curve that is strongly skewed to the right. AppendixLO1
-
Southwestern Fashions, Inc. which uses a job-order costing system had two jobs in process at the start of the year: job no. 101 ($168,000) and job no. 102 ($107,000). The following information is...
-
10.You have the alternative of paying for university fees today for a payment of $15,000 or, you can select a payment plan where you pay $8,000 in 6 months from today and another $12,000 in exactly...
-
Name the octahedral complex ions (a) cis-[CrCl 2 (NH 3 ) 4 ] + , (b) trans-[Cr(NH 3 ) 2 (N-NCS) 4 ] , (c) [Co(C 2 O 4 )(en) 2 ] + .
-
The hydrated chromium chloride that is available commercially has the overall composition CrCl 3 6H 2 O. On boiling a solution, it becomes violet and has a molar electrical conductivity similar to...
-
Kathryn Marley owns and operates the largest Mercedes- Benz auto dealership in Pittsburgh. In the past 36 months, her sales have ranged from a low of 6 new cars to a high of 12 new cars, as reflected...
-
The relationship between income and savings, let's look back to the recent credit crisis that sent our economy into the greatest financial crisis since the Great Depression. Watch this short video...
-
Jos Lpez has $15,000 in a 6-year certificate of deposit (CD) that pays a guaranteed annual rate of 4%. Create a timeline showing when the cash flows will occur. (6 points) 2. Oliver Lpez deposits...
-
PROBLEM SET #2 At a large urban college, about half of the students live off campus in various arrangements, and the other half live on campus. Is academic performance dependent on living...
-
Post a compelling argument stating whether leaders are born, made, or a combination of both. Drawing from the discussion of the two current peer-reviewed articles you identified, support your...
-
Unicorn Inc. builds commercial jets and calculate the cost for each jet. For each item below, indicate whether it would be most likely classified as direct labor (DL); direct materials (DM);...
-
Refer to the Journal of Accounting Public Policy (Vol. 34, 2015) study of the impact of executive networking on firm performance, Exercise 2.101. Recall that firm performance was measured as annual...
-
Compile data on consumption and expenditures for the following categories in 30 different countries: (1) food and beverages, (2) clothing and footwear, (3) housing and home operations, (4) household...
-
Suggest products when Me 3 Sb reacts with the following reagents: (a) B 2 H 6 ; (b) H 2 O 2 ; (c) Br 2 ; (d) Cl 2 followed by treatment with MeLi; (e) MeI; (f) Br 2 followed by treatment with Na[OEt].
-
(a) In what ways do the solid state structures of ( 5 -C 5 R 5 ) 2 Sn for R = H, Me and Ph differ? (b) In the solid state structure of ( 5 -C 5 Me 5 ) 2 Mg, the two cyclopentadienyl rings are...
-
Suggest products when Et 3 SnCl reacts with the following reagents: (a) H 2 O; (b) Na[Cp]; (c) Na 2 S; (d) PhLi; (e) Na.
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


