Which description in the second list below can be correctly matched to each compound in the first
Question:
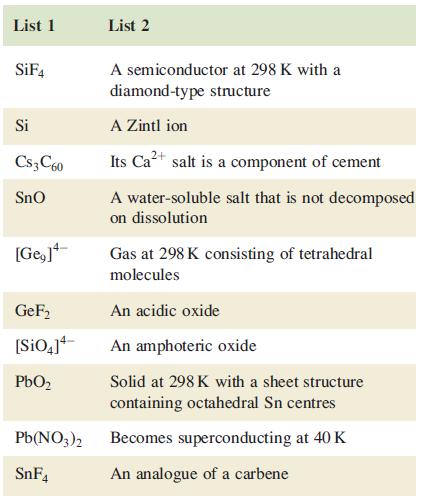
Which description in the second list below can be correctly matched to each compound in the first list? There is only one match for each pair.
Transcribed Image Text:
List 1 SiF4 Si Cs3C60 SnO [Ge,]+ GeF₂ [SiO4]¹ PbO₂ Pb(NO3)2 SnF4 List 2 A semiconductor at 298 K with a diamond-type structure A Zintl ion Its Ca²+ salt is a component of cement A water-soluble salt that is not decomposed on dissolution Gas at 298 K consisting of tetrahedral molecules An acidic oxide An amphoteric oxide Solid at 298 K with a sheet structure containing octahedral Sn centres Becomes superconducting at 40 K An analogue of a carbene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Lets match the compounds in List 1 with their corre...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which description in the second list below can be correctly matched to each element or compound in the first list? There is only one match for each pair. List 1 So [S08]- [S] SF NaO [S06] PbS HO HSO...
-
Which description in the second list below can be correctly matched to each element or compound in the first list? There is only one match for each pair. List 1 HCIO4 CaF 105 CIO [BrF] [IF] HOCI C6H6...
-
The first list below contains words or phrases, each of which has a partner in the second list, e.g. sodium in the first list can be matched with metal in the second list. Match the partners; there...
-
In what significant way do financial audits in government and not-for-profit organizations differ from those carried on in businesses? Your answer should also address the purpose of performance...
-
Solway Company is a sole proprietorship whose owner, Joe Solway, has an equity interest of $50,000. Had Solway been a partnership rather than a sole proprietorship, and the two equal partners were...
-
You have a Windows Server 2016 server named ServerA. ServerA is located on the perimeter network and only uses an inbound connection.TCP port 443 is allowed to connect ServerA from the internet. You...
-
Father, Inc., buys 80 percent of the outstanding common stock of Sam Corporation on January 1, 2009, for $680,000 cash. At the acquisition date, Sams total fair value was assessed at $850,000...
-
The following tasks are to be performed on an assembly line: The workday is seven hours long. Demand for completed product is 750 per day. a. Find the cycle time required to produce 750 units per...
-
The BEST source for general information about an industry and the names of officers and directors in companies in that industry is Select one: O a. The Wall Street Journal. O b. A directory for the...
-
Account for the fact that when aqueous solution of KCN is added to a solution of aluminium sulfate, a precipitate of Al(OH) 3 forms.
-
Comment on the following observations: (a) The pyroxenes CaMgSi 2 O 6 and CaFeSi 2 O 6 are isomorphous; (b) The feldspar NaAlSi 3 O 8 may contain up to 10% of CaAl 2 Si 2 O 8 ; (c) The mineral...
-
Argue that Use an argument similar to the one used to establish Equation (4.1). T7 n 1 n 1,n2. ,nr ni,n2,,n ni,n2 1,...,Hr J i n). 2 ni, n2.,Mr 7
-
Briefly explain the difference between a k-factor model and the capital asset pricing model
-
Refer to the cost data, Picture below. Take off the square feet of wall forms and cubic yards of ready mix concrete for the walls of the elevator pit. Determine the total material and labor cost for...
-
possible Submit quiz A researcher studies water clarity at the same location in a lake on the same dates during the course of a year and repeats the measurements on the same dates 5 years later. The...
-
A liquid hydrocarbon mixture was made by adding 295 kg of benzene, 289 kg of toluene and 287 kg of p-xylene. Assume there is no change of volume upon mixing, i.e., Vmix=0 , in order to determine: 1....
-
b) Maseru Development Bank has R850 million credit with Matsieng Hydroelectric Power, with a maturity of eighteen months. The expected loss for Maseru Development Bank is R22 million, and the...
-
In the study of personality, there are three important properties which are of central importance. Describe all three properties and explain why they are so important for marketing and consumer...
-
Digital Fruit is financed solely by common stock and has outstanding 25 million shares with a market price of $10 a share. It now announces that it intends to issue $160 million of debt and to use...
-
Identify the B-containing compounds A, B, and C. BF3 CaF2 C LiAlH4 heat A HO B
-
Given NaBH 4 , a hydrocarbon of your choice, and appropriate ancillary reagents and solvents, give formulas and conditions for the synthesis of (a) B(C 2 H 5 ) 3 , (b) Et 3 NBH 3 .
-
The rate constants for the formation of [CoX(NH 3 ) 5 ] 2+ from [Co(NH 3 ) 5 (OH 2 )] 3+ for X = Cl ,Br ,N 3 , and SCN differ by no more than a factor of 2. What is the mechanism of the...
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


