Question: With reference to Box 23.2, develop a qualitative bonding scheme for ( 5 -Cp) 2 Fe. Box 23.2. THEORY Box 23.2 Bonding in cyclopentadienyl complexes:
With reference to Box 23.2, develop a qualitative bonding scheme for (η5-Cp)2Fe.
Box 23.2.
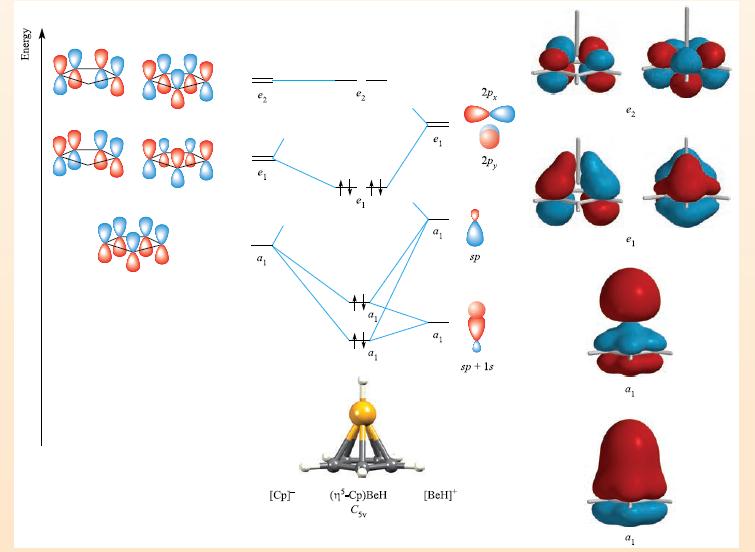
THEORY Box 23.2 Bonding in cyclopentadienyl complexes: -mode If all five C atoms of the cyclopentadienyl ring interact with the metal atom, the bonding is most readily described in terms of an MO scheme. Once the o-bonding framework of the [Cp] ligand has been formed, there is one 2p, atomic orbital per C atom remaining, and five combinations are possible. The MO diagram below shows the formation of (n-Cp)BeH (Csv), a model compound that allows us to see how the [n-Cp] ligand interacts with an s-or p-block metal fragment. For the formation of the [BeH]* fragment, we can use an sp hybridization scheme. One sp hybrid points at the H atom and the other points at the Cp ring. Using the methods from Chapter 5, the orbitals of the [BeH] unit are classified as having a, or e, symmetry within the Csv point group. To work out the 7-orbitals of the [Cp] ligand, we first determine how many C 2p, orbitals are unchanged by each symmetry operation in the Cs, point group (Appendix 3). The resultant row of characters is: 2C5 2C3 E 5 00 Say 1 This row can be obtained by adding the rows of characters for the A, E and E representations in the C5, character table. Thus, the five 7-orbitals of [Cp] possess a, e, and e symme- tries. By applying the methods described in Chapter 5, the wavefunctions for these orbitals can be determined. The orbi- tals are shown schematically on the left-hand side of the diagram. The MO diagram is constructed by matching the symmetries of the fragment orbitals. Mixing can occur between the two a orbitals of the [BeH] fragment. Four bonding MOs (a, and e) result. The e [Cp] orbitals are non-bonding with respect to Cp-BeH interactions. (Anti- bonding MOs have been omitted from the diagram.) Eight electrons are available to occupy the a and e MOs. Repre- sentations of the a, e, and e MOs are shown at the right- hand side of the figure: the e, set possesses Be-C bonding character, while both a MOs have Be-C and Be-H bonding character. Bonding in cyclopentadienyl complexes of d-block metals (see Chapter 24) can be described in a similar manner but must allow for the participation of metal d-orbitals.
Step by Step Solution
3.37 Rating (147 Votes )
There are 3 Steps involved in it
Here is a qualitative bonding scheme for 5Cp2Fe 1 Cyclopentadienyl Ligands Cp Each cyclopentadienyl ... View full answer

Get step-by-step solutions from verified subject matter experts


