(a) Derive and in terms of measurable properties. (b) dH = dU + d(PV) from the definition...
Question:
(a) Derive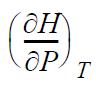
and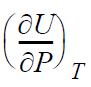
in terms of measurable properties.
(b) dH = dU + d(PV) from the definition of H. Apply the expansion rule to show the difference between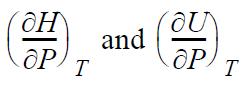
is the same as the result from part (a).
Transcribed Image Text:
он OP T
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:
Students also viewed these Engineering questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family. The Incisors own a rental beach house in Hawaii. The beach house was rented for the full year during 2012...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Define price and name the various types of prices described in this chapter.
-
Consider a small country that exports steel. Suppose that a pro-trade government decides to subsidize the export of steel by paying a certain amount for each ton sold abroad. How does this export...
-
As the manager of a small lodging operation, you have decided to implement a mandatory tip pool policy in the bar and the restaurant of your operation. Your reason for doing this is to lower labor...
-
Understand the strategic role of human resources in international business. LO.1
-
In a pure exchange economy with two goods, G and H, the two traders have Cobb-Douglas utility functions. Suppose that Tony's utility function is Ut = GtHt and Margaret's utility function is Um =...
-
do you agree with the statement it audit should be considered absolute because it implies a distinction between regular auditors and auditors who examine computerized accounting information system...
-
In Chapter 2, internal energy of condensed phases was stated to be more weakly dependent on pressure than enthalpy. This problem evaluates that statement. (a) Derive in terms of measurable...
-
Express the following in terms of U, H, S, A, and their derivatives. (RT), V (2(G/(RT)) OT
-
A one-year American call option on silver futures has an exercise price of $9.00. The current futures price is $8.50, the risk-free rate of interest is 12% per annum, and the volatility of the...
-
The State of Confusion Legislature passes the following statute: "The State Health Commissioner, when in their opinion, there is sufficient covid - 1 9 vaccine that has been approved by the Federal...
-
Case 1 Baum Co. has two processing departments: Fabrication and Assembly. In the Fabrication Department, metal is cut and formed into various components, which are then transferred to Assembly. The...
-
Your earlier Personal Leadership Assessment, you looked at two areas of your leadership experience, those who led you and those you led. You will again address these two items in your Personal...
-
What is required in this situation: Content slides explaining the qualitative and quantitative steps necessary in conducting a sensitivity analysis. How can a project's risk be incorporated into a...
-
What is "marketing"? What is the difference between "marketing" and the "marketing process"?is it different in your home country vs. North america? Q2. What is the difference between "demand",...
-
Krafty Kris, Inc., discovered the following errors after the 2017 financial statements were issued: a. A major supplier shipped inventory valued at $8,550 to Krafty Kris on consignment. This...
-
United Business Forms capital structure is as follows: Debt ............................................ 35% Preferred stock ........................... 15 Common equity .......................... 50...
-
For a 0.1 M aqueous solution of sodium acetate, Na + CH 3 CO - 2 , one mass balance is simply [Na + ] = 0.1 M. Write a mass balance involving acetate.
-
(a) Following the example of Mg(OH) 2 in Section 7-5, write the equations needed to find the solubility of Ca(OH) 2 . Include activity coefficients where appropriate. Equilibrium constants are in...
-
Look up the equilibrium constant for the ion-pairing reaction in Appendix J. (a) Use the systematic treatment of equilibrium to find [Zn 2+ ] in 0.010 F ZnSO4. Neglect activity coefficients and any...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...

Study smarter with the SolutionInn App


