Ammonia is a weak base, as indicated by the pK a , A and pK a ,
Question:
Ammonia is a weak base, as indicated by the pKa,A and pKa,B values in Table 18.2. Determine the percentage of NH3 dissociated at pH 7 and pH 1.5 when the apparent amount of NH3 in aqueous solution is 0.15 m. Assume ideal solutions.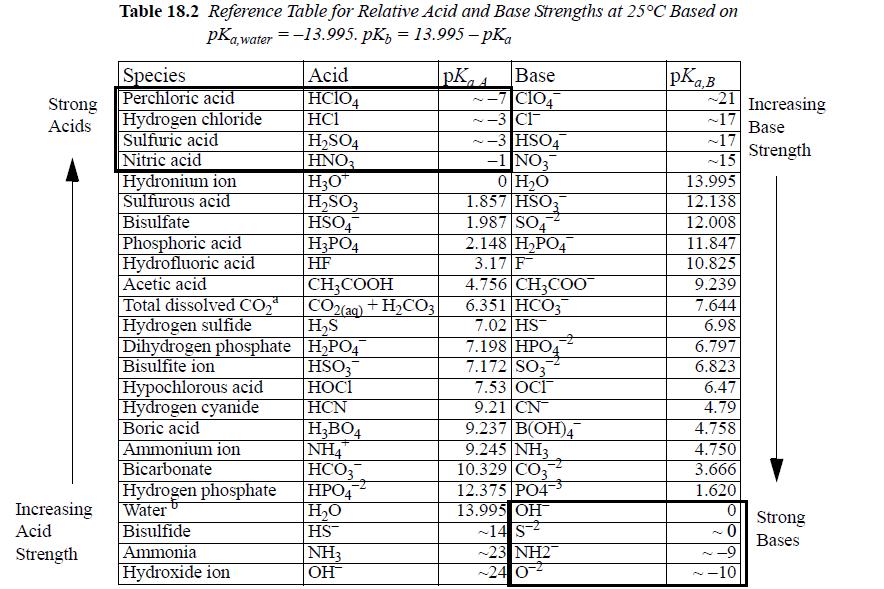
Transcribed Image Text:
Strong Acids Increasing Acid Strength Table 18.2 Reference Table for Relative Acid and Base Strengths at 25°C Based on pKa,water = -13.995. pKz 13.995-pka Species Perchloric acid Hydrogen chloride Sulfuric acid Nitric acid Hydronium ion Sulfurous acid Bisulfate Phosphoric acid Hydrofluoric acid Acetic acid Total dissolved CO₂ Hydrogen sulfide Dihydrogen phosphate Bisulfite ion Hypochlorous acid Hydrogen cyanide Boric acid Ammonium ion Bicarbonate Hydrogen phosphate Water Bisulfide Ammonia Hydroxide ion Acid HCIO4 HC1 H₂SO4 HNO3 H3O™ H₂SO3 HSO4 H3PO4 HF CH3COOH CO2(aq) + H₂CO3 H₂S H₂PO4 HSO3 НОСI HCN H₂BO4 |NH4 HCO3 HPO4 H₂O HS™ NH3 OH PKA Base ~-7 C104 ~-3 Cl -3 HSO4 -1 NO3 0 H₂O 1.857 HSO3 1.987 SO4 2.148 H₂PO4 3.17 F 4.756 CH3COO 6.351 HCO3 7.02 HS 7.198 HPO4 7.172 SO3 7.53 OCI 9.21 CN 9.237 B(OH)4 9.245 NH3 10.329 CO3 12.375 PO4³ 13.995 OH™ -14 S-2 ~23 NH2 -24 0² pKa,B 21 Increasing ~17 Base Strength ~17 ~15 13.995 12.138 12.008 11.847 10.825 9.239 7.644 6.98 6.797 6.823 6.47 4.79 4.758 4.750 3.666 1.620 0 ~0 -9 -10 N Strong Bases
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
To determine the percentage of ammonia NH dissociated at a given pH level we will need to use the gi...View the full answer

Answered By

Muqadas Javed
I am a mentor by profession since seven years. I have been teaching on online forums and in universities. Teaching is my passion therefore i always try to find simple solution for complicated problems or task grasp them so that students can easily grasp them.I will provide you very detailed and self explanatory answers and that will help you to get good grade. I have two slogans: quality solution and on time delivery.
4.60+
24+ Reviews
144+ Question Solved
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:
Students also viewed these Engineering questions
-
A GT cell will machine the components for a family of parts. The parts come in several different sizes and the cell will be designed to quickly change over from one size to the next. This will be...
-
Trish's bookkeeper has provided the book-basis fixed asset roll forward to be used in computing the depreciation (see separate tab). For tax purposes, Trish elected out of bonus depreciation in all...
-
Part 1 a. Ammonia, NH 3 , is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this...
-
______________ is an approach to doing business that attempts to maximize an organization's competitiveness through the continual improvement of the quality of its products, services, people,...
-
Compare and contrast the four ways managers make decisions.
-
The text discusses four principles of forecasting. Name and describe each.
-
9. When does a treasury stock transaction affect the investment account? How is the effect adjusted?
-
The law firm of Adams, Babcock, and Connors is located in the DallasFort Worth metroplex. Randall Adams is the senior and founding partner in the firm. John Babcock has been a partner in the firm for...
-
Edit View Go Tools Window Help Session 16 Pricing Exercise-2.pdf (page 3 of 4) - Edited Q Search Exercise 3: Target Profit Pricing As the term suggests, target profit pricing is designed to determine...
-
(a) Compute the freezing point depression for an aqueous solutions that is 3 wt% NaCl. (b) Compute the boiling point elevation for an aqueous solutions that is 3 wt% NaCl. (c) Compute the osmotic...
-
For nonylglucoside, NG, thermodynamic data for demicellization in water are presented in problem 17.26. Model the micelle reaction as n S M n where S is free surfactant and M n is a micelle. Treat...
-
Refer to the amortization schedule presented in BE15-8 for Chiasson Corp. (a) Assuming Chiasson redeems these bonds at 100 on January 1, 2015, after the interest has been paid, prepare the journal...
-
Describe how the ideas within this Preamble align with your own personal values and career goals as a social work professional.
-
Maersk managers are adapting with various types of industry and market changes to nurture a contemporary approach to management . Analyze the various contemporary management practices of Maersk.
-
then P Let p 2 be an integer such that for any a, b integers, if divides a or p divides b. Show that p is prime. P divides ab
-
For Q2, you will use logistic regression to segment customers into two classes. This question is adapted from a Kaggle contest to evaluate current customers for an auto dealership that is opening a...
-
Figure how to fill out the rest Cash Budget 2018 Cash Balance, Beginning $ Q1 50,000 Q2 Q3 Q4 Cash Collections Cash Available 2,599,218 2,649,218 Manufacturing Outflows: Direct Materials 1,645,183...
-
In a Pew Research Center survey of Internet users, 3732 respondents say that they use social networking sites and 1380 respondents say that they do not use social networking sites. What is the...
-
In Problem use geometric formulas to find the unsigned area between the graph of y = f(x) and the x axis over the indicated interval. f(x) = x + 5; [0, 4]
-
Calculate the pH when 25.0 mL of 0.020 0 M 2-aminophenol have been titrated with 10.9 mL of 0.015 0 M HClO 4 .
-
Find the equilibrium constant for the reaction of MES (Table 8-2) with NaOH.
-
Find the equilibrium constant for the reaction of MES (Table 8-2) with NaOH. Table 8-2 A(pKa/AT (K) Formula Name Structure u = 0.1 M mass CH,CO,H H,NCCH,NH - (CO,H) 1.59 190.15...
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


