Find the equilibrium constant for the reaction of MES (Table 8-2) with NaOH. Table 8-2 A(pKa/AT (K)
Question:
Find the equilibrium constant for the reaction of MES (Table 8-2) with NaOH.
Table 8-2
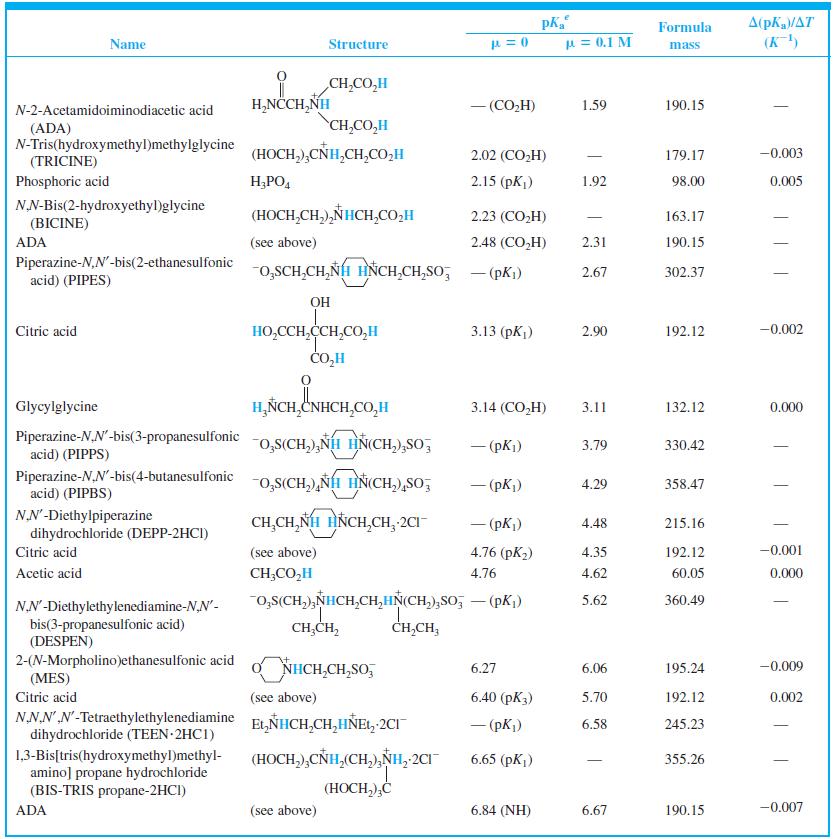
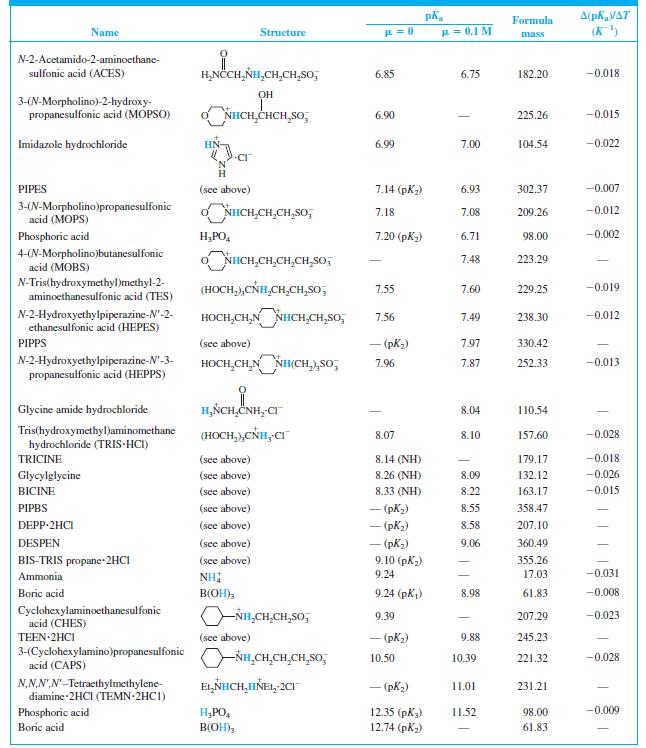
Transcribed Image Text:
A(pKa/AT (K) Formula Name Structure u = 0.1 M mass CH,CO,H H,NCCH,NH - (CO,H) 1.59 190.15 N-2-Acetamidoiminodiacetic acid CH,CO,H (ADA) N-Tris(hydroxymethyl)methylglycine (TRICINE) (HOCH,),CÑH,CH,CO,H 2.02 (CO,H) 179.17 -0.003 Phosphoric acid H;PO, 2.15 (pK) 1.92 98.00 0.005 NN-Bis(2-hydroxyethyl)glycine (HOCH,CH,),NHCH,CO,H 2.23 (CO,H) 163.17 | (BICINE) ADA (see above) 2.48 (СO,H) 2.31 190.15 Piperazine-N,N'-bis(2-ethanesulfonic acid) (PIPES) -o,SCH,CH,NH HNCH,CH,So; - (pKi) 2.67 302.37 OH HO,CCH,CCH,CO,H CO,H Citric acid 3.13 (pKj) 2.90 192.12 -0.002 Glycylglycine H,ÑCH,CNHCH,CO,H 3.14 (CO,H) 3.11 132.12 0.000 Piperazine-N,N'-bis(3-propanesulfonic acid) (PIPPS) "o,S(CH,),NÍH HN(CH,),SO, - (pK) 3.79 330.42 Piperazine-N,N'-bis(4-butanesulfonic acid) (PIPBS) "0,S(CH,),ÑH HN(CH,),SO, 4.29 358.47 ('yd) – NN'-Diethylpiperazine dihydrochloride (DEPP-2HCI) CH,CH, NH HNCH,CH, 2CI - (pK) 4.48 215.16 Citric acid (see above) 4.76 (pK2) 4.35 192.12 -0.001 Acetic acid CH;CO,H 4.76 4.62 60.05 0.000 o,s(CH,),NHCH,CH,HN(CH,),So, – (pK,) 5.62 360.49 NN'-Diethylethylenediamine-N,N'- bis(3-propanesulfonic acid) (DESPEN) CH,CH, CH,CH, 2-(N-Morpholino)ethanesulfonic acid (MES) Citric acid NHCH,CH,SO, 6.27 6.06 195.24 -0.009 (see above) 6.40 (рК3) 5.70 192.12 0.002 NN.NN'-Tetraethylethylenediamine dihydrochloride (TEEN 2HC1) EL,NHCH,CH,HÑE1, 2CI - (pK) 6.58 245.23 (HOCH,),CNH,(CH,),NH, 2CI 1,3-Bis[tris(hydroxymethyl)methyl- amino] propane hydrochloride (BIS-TRIS propane-2HCI) 6.65 (рK,) 355.26 (HOCH,),Ć ADA (see above) 6.84 (NH) 6.67 190.15 -0.007
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (14 reviews)
To find the equilibrium constant for the reaction of MES 2NMorpholinoethanesulfonic acid with NaOH w...View the full answer

Answered By

Rayan Gilbert
I have been teaching since I started my graduation 3 years ago. As a student, working as Teacher/PA has been tough but made me learn the needs for student and how to help them resolve their problems efficiently. I feel good to be able to help out students because I'm passionate about teaching. My motto for teaching is to convey the knowledge I have to students in a way that makes them understand it without breaking a sweat.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Find the equilibrium constant for the reaction 2NO + O2 2NO2 from the elementary reactions in Table A.10 to answer which of the nitrogen oxides, NO or NO2; is the more stable at...
-
Find the equilibrium constant for the reaction 2NO + O2 2NO2 from the elementary reactions in Table A.11 to answer which of the nitrogen oxides, NO or NO2, is the more stable at ambient conditions?...
-
The equilibrium constant for the reaction of NH3 (aq) + H2O NH+4 + OH- is Kb = 1.479 10-5 at 5C 1.570 10 -5 at 10oC (a) Assuming H and S are constant in the interval 5 - 10C (probably a good...
-
There are major responsibilities of system administrator as listed below: o Start-up and shut down the system o Performance tuning o Managing user accounts o System security o Backup and recovery o...
-
You are asked to supervise a new hire in your firm's tax department. The person has been working on the tax return of a corporation that is a new client of the firm. Help the person put together the...
-
The process that involves monitoring equipment and facilities, and performing routine inspections and service to keep equipment and facilities reliable is known as: a) breakdown maintenance. b)...
-
On December 20, 2009, Butanta Company (a U.S. company headquartered in Miami, Florida) sold parts to a foreign customer at a price of 50,000 ostras. Payment is received on January 10, 2010. Currency...
-
What are the three categories of funds prescribed by GASB standards and which fund types are included in each? Do the three fund categories correspond precisely with the three activity categories...
-
Please show your work Wenkboxed window by be 75.000 hideg som kom 38 per sta ce este dep.co LATA NO.000 INSTRUCTION a) Prepare the A15 journal entry for the books of Wisc Duter den for Macht). Accome...
-
Ace Companys income statements for the three years 2012, 2011, and 2010 are given below (all amounts in $ millions): 1. Other income (expense) comprised the following: 2. In 2012, cost of goods sold...
-
Find the equilibrium constant for the reaction of MES (Table 8-2) with NaOH.
-
When 22.63 mL of aqueous NaOH were added to 1.214 g of cyclohexylaminoethanesulfonic acid (FM 207.29, structure in Table 8-2) dissolved in 41.37 mL of water, the pH was 9.24. Calculate the molarity...
-
Write an essay distinguishing between the four different types of banking and explain their different functions.
-
X Calculate the reaction rate at various conversions, as shown below: FAO -TA -TA 0.2 0.8 Considering that for a PFR: dx V = FAO What is the conversion reached after the 50 m of this PFR?
-
For decades, leaders have talked about flexible working options, yet only few companies were consistently using these practices prior to the global health crisis of 2020. In March 2020, organizations...
-
Manatee Corp. has developed standard costs based on a predicted operating level of 352,000 units of production, which is 80% of capacity. Variable overhead is $281,600 at this level of activity, or...
-
When leaders are facing a crisis or an opportunity, generally, they tend to fall back on the leadership style that has worked for them in the past. Discuss with examples the options that would help...
-
Data Table the pasteet dollar -X Total sales revenues 2 Number of units produced and sold 500,000 units Selling price nt. $ 230,000 te Operating Income Total Investment in assets Variable cost per...
-
The management of an engineering consultant firm is considering the possibility of setting up its own market research department rather than continuing to use the services of a market research firm....
-
Study the pictures/images below. Obviously these was focus on LT sociology, anthropology and poltical science. Try to do some analysis by finding clues that are synonymous with the main concepts....
-
For the system in Problem P6.6, suppose your design requirement was to minimize the force necessary to hold the gate closed. Would you rather put the hinge at the top of the gate or at the bottom?...
-
Make an order-of-magnitude estimate for a safety floatation device that can be used by children who are playing in a swimming pool. The design concept is for one inflatable, annular, plastic balloon...
-
An ancient kings supposedly golden crown had a mass of 3 kg, but it was actually made by a dishonest metal smith from an equal mix of gold (1.93 10 4 kg/m 3 ) and silver (1.06 10 4 kg/m 3 ). (a)...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


