When 22.63 mL of aqueous NaOH were added to 1.214 g of cyclohexylaminoethanesulfonic acid (FM 207.29, structure
Question:
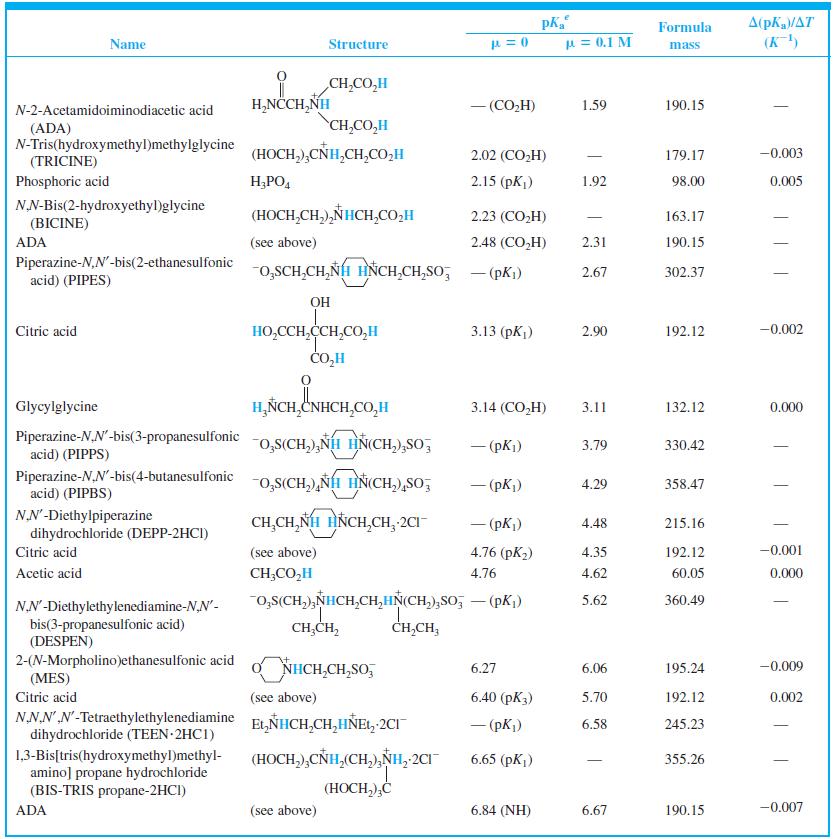
When 22.63 mL of aqueous NaOH were added to 1.214 g of cyclohexylaminoethanesulfonic acid (FM 207.29, structure in Table 8-2) dissolved in 41.37 mL of water, the pH was 9.24. Calculate the molarity of the NaOH.
Table 8-2
Transcribed Image Text:
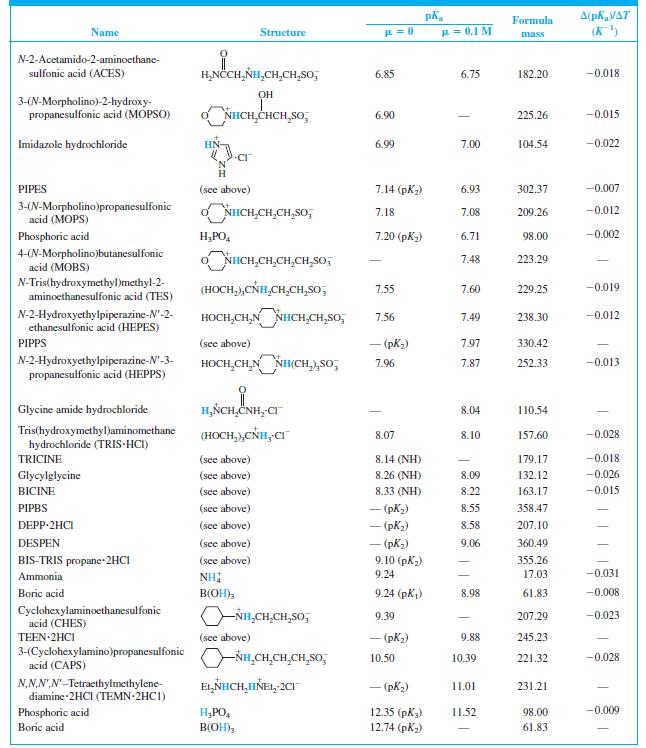
A(pKa/AT (K) Formula Name Structure u = 0.1 M mass CH,CO,H H,NCCH,NH - (CO,H) 1.59 190.15 N-2-Acetamidoiminodiacetic acid CH,CO,H (ADA) N-Tris(hydroxymethyl)methylglycine (TRICINE) (HOCH,),CÑH,CH,CO,H 2.02 (CO,H) 179.17 -0.003 Phosphoric acid H;PO, 2.15 (pK) 1.92 98.00 0.005 NN-Bis(2-hydroxyethyl)glycine (HOCH,CH,),NHCH,CO,H 2.23 (CO,H) 163.17 | (BICINE) ADA (see above) 2.48 (СO,H) 2.31 190.15 Piperazine-N,N'-bis(2-ethanesulfonic acid) (PIPES) -o,SCH,CH,NH HNCH,CH,So; - (pKi) 2.67 302.37 OH HO,CCH,CCH,CO,H CO,H Citric acid 3.13 (pKj) 2.90 192.12 -0.002 Glycylglycine H,ÑCH,CNHCH,CO,H 3.14 (CO,H) 3.11 132.12 0.000 Piperazine-N,N'-bis(3-propanesulfonic acid) (PIPPS) "o,S(CH,),NÍH HN(CH,),SO, - (pK) 3.79 330.42 Piperazine-N,N'-bis(4-butanesulfonic acid) (PIPBS) "0,S(CH,),ÑH HN(CH,),SO, 4.29 358.47 ('yd) – NN'-Diethylpiperazine dihydrochloride (DEPP-2HCI) CH,CH, NH HNCH,CH, 2CI - (pK) 4.48 215.16 Citric acid (see above) 4.76 (pK2) 4.35 192.12 -0.001 Acetic acid CH;CO,H 4.76 4.62 60.05 0.000 o,s(CH,),NHCH,CH,HN(CH,),So, – (pK,) 5.62 360.49 NN'-Diethylethylenediamine-N,N'- bis(3-propanesulfonic acid) (DESPEN) CH,CH, CH,CH, 2-(N-Morpholino)ethanesulfonic acid (MES) Citric acid NHCH,CH,SO, 6.27 6.06 195.24 -0.009 (see above) 6.40 (рК3) 5.70 192.12 0.002 NN.NN'-Tetraethylethylenediamine dihydrochloride (TEEN 2HC1) EL,NHCH,CH,HÑE1, 2CI - (pK) 6.58 245.23 (HOCH,),CNH,(CH,),NH, 2CI 1,3-Bis[tris(hydroxymethyl)methyl- amino] propane hydrochloride (BIS-TRIS propane-2HCI) 6.65 (рK,) 355.26 (HOCH,),Ć ADA (see above) 6.84 (NH) 6.67 190.15 -0.007
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
So the reaction between acid and base is 11 mole ratio Volume of NaOH solution V 1 2263mL concent...View the full answer

Answered By

Parvathy D nair
Hi..it s me PARVATHY ..hails from KERALA ..i am a professional tutor and working in online platform.i have a experience of 2 years and solved more than 10000 answers through online platforms.i am a professional anchor also.i am a degree holder and doing bsc in zoology.currently waiting for MBBS admission.teaching is my passion.it s not only a job for me,,it s a devine profession.i see all my Students as my little brother and sisters..
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
When 5.00 mL of 0.1032 M NaOH were added to 0.1123 g of alanine (FM 89.093) in 100.0 mL of 0.10 M KNO3, the measured pH was 9.57. Use activity coefficients to find pK2 for alanine. Consider the ionic...
-
When 100.0 mL of a weak acid were titrated with 0.093 81 M NaOH, 27.63 mL were required to reach the equivalence point. The pH at the equivalence point was 10.99. What was the pH when only 19.47 mL...
-
When an ?-hydroxy amide is treated with Br2 in aqueous NaOH under Hofmann rearrangement conditions, loss of CO2 occurs and a chain-shortened aldehyde is formed. Propose a mechanism. NH2 NH3 Br2...
-
Carlberg Company has two manufacturing departments, Assembly and Painting. The Assembly department started 11,000 units during November. The following production activity in both units and costs...
-
A taxpayer works for a law firm. You are working on her tax return. She tells you that she does not have any receipts for her daily parking because the firm pays the parking lot directly for an...
-
Given the probabilities that follow for Ronny Richardsons print shop, find the expected breakdown cost. L01 NUMBER OF BREAKDOWNS 0 1 2 3 DAILY FREQUENCY 0.3 0.2 0.2 0.3 The cost per breakdown is $10.
-
New Colony Corporation (a U.S. company) made a sale to a foreign customer on September 15, 2009, for 100,000 foreign currency units (FCU). It received payment on October 15, 2009. The following...
-
Knitline Inc. produces high-end sweaters and jackets in a single factory. The following information was provided for the coming year. A sales commission of 5% of sales is paid for each of the two...
-
Which of the following statement describe a sole proprietorship? a. The sole proprietorship business has unlimited capital compared to partnership b. The owner of sole proprietorship has less...
-
Amanda Boleyn, an entrepreneur who recently sold her start-up for a multi-million-dollar sum, is looking for alternate investments for her newfound fortune. She is considering an investment in wine,...
-
Find the equilibrium constant for the reaction of MES (Table 8-2) with NaOH. Table 8-2 A(pKa/AT (K) Formula Name Structure u = 0.1 M mass CH,CO,H H,NCCH,NH - (CO,H) 1.59 190.15...
-
Why is the equivalence-point pH necessarily below 7 when a weak base is titrated with strong acid?
-
Remembering that the amino acid side chains projecting from each polypeptide backbone in a sheet point alternately above and below the plane of the sheet (see Figure 413A), consider the following...
-
Figure < 4 ft/s 45 0.75 ft 3 ft/s 1.50 ft 1 of 1 < Part A Determine the velocity of point A on the rim of the gear at the instant shown.(Figure 1) Enter the x and y components of the velocity...
-
what ways can leaders facilitate cognitive reframing and emotional regulation techniques to promote constructive conflict resolution ?
-
What is the level of sales needed to achieve a 10% return on an investment of $10,000,000 for a restaurant (the restaurant has main products it sells: food, beverage and gift shop items) and cover...
-
1. An online computer assembling mobile phone Application provides interfaces for end users to assemble computers by selecting computer accessories with different configurations from different...
-
1. (# 3.21, Text) Plot the longitudinal and transverse coefficients of thermal expansion for a unidirectional glass-polyester composite as functions of fiber volume fraction. Assume the following...
-
In a classic research paper, Hamilton (1987) illustrated the multicollinearity problem with an example using the data shown in the next table. The values of x1, x2, and y in the table represent...
-
Why do bars offer free peanuts?
-
Scuba divers carry ballast weights to have neutral buoyancy. At that condition, the buoyancy force on the diver exactly balances weight, and there is no tendency either to float toward the surface or...
-
Examine the transition between the laminar and turbulent flows of water by sketching the stream of water that exits from a faucet (without an aerator) or hose (without a nozzle). You can control the...
-
Water flows through a 5-cm-diameter pipe at the average velocity of 1.25 m/s. (a) In the dimensions of L/s, what is the volumetric flow rate? (b) If the diameter of the pipe is reduced by 20% at a...
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


