Beginning with the untransformed Gibbs energies of formation, document the intermediate calculations for the value of apparent
Question:
Beginning with the untransformed Gibbs energies of formation, document the intermediate calculations for the value of apparent Gibbs energy of formation of ADP at the conditions of Example 18.10, using the extended Debye-Hückel activity coefficient model and transformed Gibbs energies. Also calculate the distribution of each species.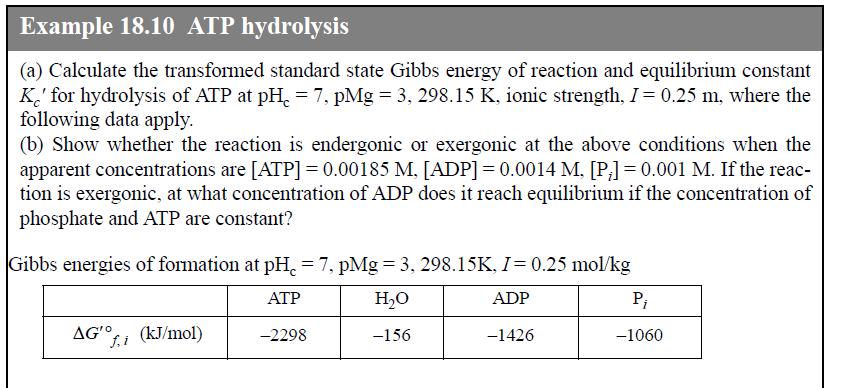
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





