Estimate C P , C V , and the difference C P - C V in (J/mol-K)
Question:
Estimate CP, CV, and the difference CP - CV in (J/mol-K) for liquid n-butane from the following data.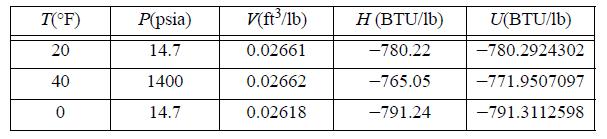
Transcribed Image Text:
T(°F) 20 40 0 P(psia) 14.7 1400 14.7 V(ft³/1b) 0.02661 0.02662 0.02618 H (BTU/lb) -780.22 -765.05 -791.24 U(BTU/1b) -780.2924302 -771.9507097 -791.3112598
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
To estimate the heat capacities at constant pressure CP and at constant volume CV for liquid nbutane we would typically use the following relationships CP dHdTP CV dUdTV where H is enthalpy U is internal energy and T is temperature This requires derivatives of H and U with respect to temperature at constant pressure or volume respectively Unfortunately the data provided doesnt have multiple enthalpy H or internal energy U values at the same pressure or volume to calculate these derivatives directly Since we cant make a direct calculation without additional data or assumptions we have to estimate based on the data at hand We do see data at two different temperatures for the same pressure 147 psia which can be used to crudely estimate CP Assuming the change in enthalpy H is proportional to the change in temperature T we can make the following approximation CP HT where H and T must be in consistent units We have to convert temperature to Kelvin or degrees Celsius for the calculation as entropy and enthalpy changes are typically measured with these units First convert the temperatures from Fahrenheit to Kelvin K TK TF 32 59 27315 T1K 20F 32 59 27315 266483 K T2K 0F 32 59 27315 255372 K Now find the enthalpy change H in the consistent units of Jmol Note 1 BTUlb is approximately 2326 Jg H1 78022 BTUlb H2 79124 BTUlb To convert BTU to J and then from per pound to per mole we need the molecular weight of nbutane which is approximately 5812 gmol H H2 H1 2326 Jg 453592 glb 1 mol5812 g H 79124 78022 2326 453592 5812 Jmol H 1102 2326 453592 5812 Jmol H 1813502 Jmol Now find the temperature change T in Kelvin T T1 T2 266483 K 255372 K 11111 K Now we can estimate CP CP HT 1813502 Jmol 11111 K 163345 JmolK To estimate CV we would need similar data at constant volume which is not provided in the table The difference between CP and CV for an ideal gas is given by the gas constant R which is approximately 8314 JmolK However as were dealing with a liquid and not an ideal gas this relationship ...View the full answer

Answered By

Saikumar Ramagiri
Financial accounting:- Journal and ledgers, preparation of trail balance and adjusted trail balance Preparation of income statement, retained earning statement and balance sheet Banks reconciliation statements Financial statement analysis Cash flow statement analysis (both direct and indirect methods) All methods of Depreciations Management Accounting:- Ratios Budgeting control Cash budget and production budget Working capital management Receivable management Costing:- Standard and variance costing Marginal costing and decision making Cost-volume-profit analysis Inventory management (LIFO, FIFO) Preparation and estimation of cost sheet Portfolio management:- Calculation of portfolio standard deviation or risk Calculation of portfolio expected returns CAPM, Beta Financial management:- Time value of money Capital budgeting Cost of capital Leverage analysis and capital structure policies Dividend policy Bond value calculations like YTM, current yield etc International finance:- Derivatives Futures and options Swaps and forwards Business problems Finance problems Education (mention all your degrees, year awarded, Institute/University, field(s) of major): Education Qualification Board/Institution/ University Month/Year of Passing % Secured OPTIONALS/ Major ICWAI(inter) ICWAI inter Pursuing Pursuing - M.com(Finance) Osmania University June 2007 65 Finance & Taxation M B A (Finance) Osmania University Dec 2004 66 Finance & Marketing. B.Com Osmania University June 2002 72 Income Tax, Cost & Mgt, Accountancy, Auditing. Intermediate (XII) Board of Intermediate May 1999 58 Mathematics, Accountancy, Economics. S S C (X) S S C Board. May 1997 74 Mathematics, Social Studies, Science. Tutoring experience: • 10 year experience in online trouble shooting problems related to finance/accountancy. • Since 6 Years working with solution inn as a tutor, I have solved thousands of questions, quick and accuracy Skills (optional): Technical Exposure: MS Office, SQL, Tally, Wings, Focus, Programming with C Financial : Portfolio/Financial Management, Ratio Analysis, Capital Budgeting Stock Valuation & Dividend Policy, Bond Valuations Individual Skills : Proactive Nature, Self Motivative, Clear thought process, Quick problem solving skills, flexible to complex situations. Achievements : 1. I have received an Award certificate from Local Area MLA for the cause of getting 100% marks in Accountancy during my Graduation. 2. I have received a GOLD MEDAL/Scholarship from Home Minister in my MBA for being the “Top Rank student “ of management institute. 3. I received numerous complements and extra pay from various students for trouble shooting their online problems. Other interests/Hobbies (optional): ? Web Surfing ? Sports ? Watching Comics, News channels ? Miniature Collection ? Exploring hidden facts ? Solving riddles and puzzles
4.80+
391+ Reviews
552+ Question Solved
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:
Students also viewed these Engineering questions
-
Estimate the specific-heat difference cp - cv for liquid water at 15 MPa and 80C.
-
Estimate the specific-heat difference cp - cv for liquid water at 1000 psia and 150F.
-
Estimate the specific heat difference cp - cv for liquid water at 15 MPa and 80oC.
-
On January 15, Tundra Co. sold merchandise to customers for cash of $42,000 (cost $28,500). Merchandise costing $10,500 was sold to customers for $15,800 on January 17; terms 2/10, n/30. Sales...
-
The information in many of the tables in this chapter can be found in the Economic Report of the President, which appears annually. Using a recent issue of the report at your library or on the...
-
How did you feel when you switched roles and started arguing for an opposite point of view?
-
In some multicultural organizations, nationalist feelings or anti-foreigner prejudice or both are at the root of various kinds of discrimination. For instance, minority employees may be excluded...
-
During the first-year audit of Jones Wholesale Stationery, you observe that commissions amount to almost 25 percent of total sales, which is somewhat higher than in previous years. Further...
-
What is the market risk premium if the market return is 14.23% and the T-Bill rate is 3.65% ? 17.88%8.94%14.75%13.73%10.58%3.65%14.23%
-
For certain fluids, the equation of state is given by Z = 1 - b/T r . Develop an expression for the enthalpy departure function for fluids of this type.
-
Suppose that a reasonable approximation for the radial distribution function is g(r) = 0 for r < , and for r where u is the square-well potential and b = N A 3 /6. Derive an equation of state for...
-
The function dfp parse() in Fig. 6.1 works with decimal64 numbers only. Create a version that works with decimal32 numbers. Test it with the following inputs, A2 10 C6 15 -3.1415 F8 00 00 00 -inf 22...
-
Vaporization of mixtures of hexane and octane. Using the T-x-y diagram (Figure 1) on the next page, determine the temperature, amounts, and compositions of the vapor and liquid phases at 1 atm for...
-
what should p&g do to replace lafley when he retires a second time? what actions should they take to prepare for the succession?
-
What do these terms mean? What would be the currencies (one at a time) from two total UN Member States (other than the EURO, USD, JPY, GBP, or CHF). What would be the foreign currencies and how they...
-
How do social identity processes, such as categorization, identification, and comparison, influence team cohesion and performance within complex organizational environments ?
-
How do calculate sales forecast and expense forecast for several years
-
Online Auction Company (OAC) provides a platform for its users to buy and sell goods. Sellers post goods for sale and other users bid on them. They high bidder wins the auction and purchases the...
-
White Bolder Investments (WBI) You are an intern working for WBI, a large investment advisory services in Sydney. Among other regular customers, WBI has been providing advisory services for Jumbo...
-
With respect to the virial expansions, Eqs. (3.33) and (3.34), show that: where 1/V. Equation (3.33) Equation (3.34) ze, B' = ze and B = TP=0 Tp=0
-
Any equation of state valid for gases in the zero-pressure limit implies a full set of virial coefficients. Show that the second and third virial coefficients implied by the generic cubic equation of...
-
Any equation of state valid for gases in the zero-pressure limit implies a full set of virial coefficients. Show that the second and third virial coefficients implied by the generic cubic equation of...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


