Any equation of state valid for gases in the zero-pressure limit implies a full set of virial
Question:
Any equation of state valid for gases in the zero-pressure limit implies a full set of virial coefficients. Show that the second and third virial coefficients implied by the generic cubic equation of state, Eq. (3.41), are:![]() Specialize the result for B to the Redlich/Kwong equation of state, express it in reduced form, and compare it numerically with the generalized correlation for B for simple fluids, Eq. (3.61). Discuss what you find.
Specialize the result for B to the Redlich/Kwong equation of state, express it in reduced form, and compare it numerically with the generalized correlation for B for simple fluids, Eq. (3.61). Discuss what you find.
Equation (3.41)
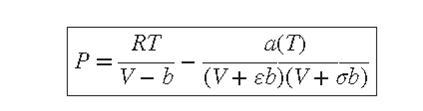
Equation (3.61)
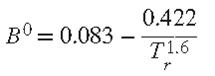
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:





