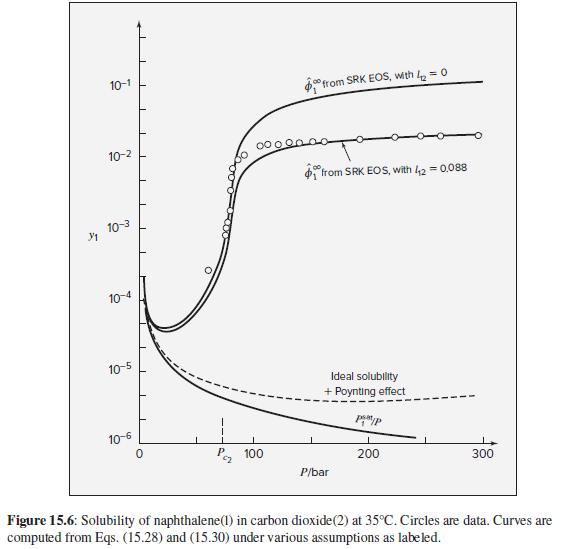
The qualitative features of SVE at high pressures shown in Fig. 15.6 are determined by the equation
Question:
The qualitative features of SVE at high pressures shown in Fig. 15.6 are determined by the equation of state for the gas. To what extent can these features be represented by the two-term virial equation in pressure, Eq. (3.36)?
Figure 15.6
Eq (3.36)

Transcribed Image Text:
10-1 °from SRK EOS, with k = 0 10-2 from SRK EOS, with 42 = 0.088 10-3 10-4 10-5 Ideal solubility + Poynting effect 10-6 P, 100 200 300 P/bar Figure 15.6: Solubility of naphthalene(1) in carbon dioxide(2) at 35°C. Circles are data. Curves are computed from Eqs. (15.28) and (15.30) under various assumptions as labeled.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (16 reviews)
The question is regarding the solubility of naphthalene in carbon dioxide at high pressures and how well this can be described by a twoterm virial equ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
What are a firm's dynamic capabilities? To what extent can managers create or "manage into existence" a firm's dynamic capabilities?
-
To what extent can personal creditors seek recovery from partnership assets?
-
To what extent can government regulate individual choices regarding sexuality?
-
A ball, which we can treat as a point charge, has a charge of +Q. This ball is 50 cm away from a ball of charge-100, which is fixed in position. The +Q ball is 30 cm vertically below, and 40 cm...
-
Excess capacity is the price we pay for production differentiation. Evaluate this statement in terms of monopolistic competition.
-
You overheard the following dialogue between Joe Ashley (a staff assistant) and Monique Estrada (his supervisor). Required: Referring to appropriate professional standards, comment on each of these...
-
How would each of the following changes tend to affect aggregate (that is, the average for all corporations) payout ratios, other things held constant? Explain your answers. a. An increase in the...
-
PlastiCo produces plastic pipe to customer specifications. Losses of less than 5 percent are considered normal because they are inherent in the production process. The company applies overhead to...
-
Please help find the manufactured cost of goods using the information below. Required information The following selected account balances are provided for Delray Mfg. $ Sales Raw materials inventory,...
-
The management of the just Like Home restaurant has asked you to analyze some of its processes. One of these processes is making a single-scoop ice cream cone. Cones can be ordered by a server (for...
-
Any equation of state valid for gases in the zero-pressure limit implies a full set of virial coefficients. Show that the second and third virial coefficients implied by the generic cubic equation of...
-
Work the preceding problem for mole fractions z 1 = 0.32, z 2 = 0.45, z 3 = 0.23.
-
Match each of the key terms above with the definition that best fits it. Memorability ____ A usability dimension concerned with how difficult it is for the user to perform a task for the first time....
-
Write out the form of the partial fraction decomposition of the function (see example). Do not determine the numerical values of the coefficients. x3 (a) x + 7x+6 9x+1 (b) (x + 1)3(x + 2) Submit...
-
You desire to make an 80% by weight vinyl acetate to 20% by weight styrene copolymer via free radical, emulsion polymerization. The r 1 and r 2 values for these monomers are 0.01 and 55,...
-
Q1)In a wheel and axle machine the diameters of the wheel and the axle are 450mm and 60mm respectively.The efficiency is 97%(0.97 per unit).When a body having a mass of 40kg is being lifted.Determine...
-
Smith & Chief Ltd. of Sydney, Australia, is a merchandising firm that is the sole distributor of a product that is increasing in popularity among Australian consumers. The company's income statements...
-
C. In lab, you measure the x & y components of a possible incompressible flow field as u = 2cxy; and where cand a are constants. v = c(a + x - y) 5. (04 pts) Short answer, what is necessary for the...
-
Presented below is information for Lieu Co. for the month of January 2022. Instructions a. Prepare an income statement using the format presented in Illustration 5.12. b. Prepare a comprehensive...
-
What is the mode?
-
Laugier and Richon (J. Chem. Eng. Data, 40:153, 1995) report the following data for the H 2 S + benzene system at 323 K and 2.010 MPa: x 1 = 0.626; y 1 = 0.986. (a) Quickly estimate the vapor-liquid...
-
Consider two gases that follow the virial equation. Show that an ideal mixture of the two gases follows the relation B = y 1 B 11 + y 2 B 22 .
-
Acrolein (C 3 H 4 O) + water exhibits an atmospheric (1 bar) azeotrope at 97.4 wt% acrolein and 52.4C. For acrolein: T c = 506 K; P c =51.6 bar; and = 0.330; MW =56. (a) Determine the value of k ij...
-
XF Ltd. Is an expanding private company in the electric trade. Accounts preparing in January 2019 included the following information: Profit Statement for the year ended 31 st December 2018 Kshs.000...
-
Check On June 15, 2021, Sanderson Construction entered into a long-term construction contract to build a baseball stadium in Washington D.C., for $340 million. The expected completion date is April...
-
Q.1 Bassem Company purchased OMR420,000 in merchandise on account during the month of April, and merchandise costing OMR $350,000 was sold on account for OMR 425,000. Required: 1. Prepare journal...

Study smarter with the SolutionInn App


