For one of the binary systems listed in Table 13.10, based on Eq. (13.19) and the Wilson
Question:
For one of the binary systems listed in Table 13.10, based on Eq. (13.19) and the Wilson equation, prepare a Pxy diagram for t = 60°C.
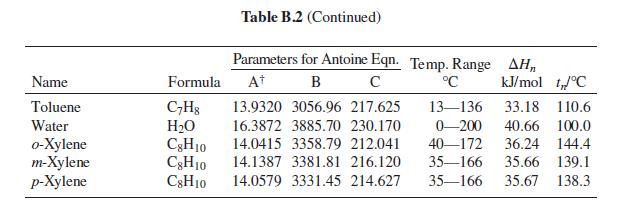
Problems 13.43 through 13.54 require parameter values for the Wilson or NRTL equation for liquid-phase activity coefficients. Table 13.10 gives parameter values for both equations. Antoine equations for vapor pressure are given in Table B.2, Appendix B.
Table 13.10
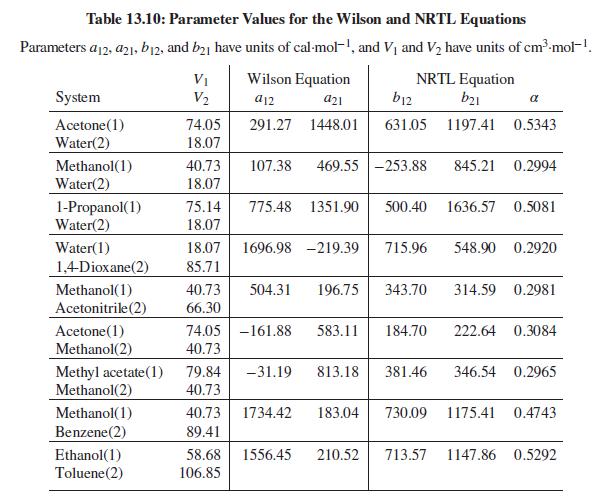
Table B.2, Appendix B.
Eq. (13.19)
![]()
Transcribed Image Text:
Table 13.10: Parameter Values for the Wilson and NRTL Equations Parameters a12, a21, b12, and baj have units of cal-mol-1, and Vị and V2 have units of cm³-mol-!. Wilson Equation NRTL Equation b12 Vị System V2 a12 b21 a Acetone(1) Water(2) Methanol(1) Water(2) 1-Propanol(1) Water(2) 74.05 291.27 1448.01 631.05 1197.41 0.5343 18.07 40.73 107.38 469.55 -253.88 845.21 0.2994 18.07 75.14 775.48 1351.90 500.40 1636.57 0.5081 18.07 18.07 1696.98 -219.39 548.90 0.2920 Water(1) 1,4-Dioxane(2) 715.96 85.71 Methanol(1) 40.73 504.31 196.75 343.70 314.59 0.2981 Acetonitrile(2) 66.30 Acetone(1) 74.05 -161.88 583.11 184.70 222.64 0.3084 Methanol(2) 40.73 Methyl acetate(1) Methanol(2) 813.18 381.46 79.84 40.73 -31.19 346.54 0.2965 Methanol(1) 40.73 1734.42 183.04 730.09 1175.41 0.4743 Benzene(2) 89.41 1556.45 Ethanol(1) Toluene(2) 58.68 210.52 713.57 1147.86 0.5292 106.85
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
The following standard costs were developed for one of the products of ABC Company: STANDARD COST CARD PER UNIT Direct materials: 20 pounds x $10 per pound .................................. $200.00...
-
For one of the companies listed in Table 5- 5 in this chapter, find five published articles in either academic journals or practitioner magazines. Prepare a report that discusses the following: a....
-
For one of the following, determine the ratio of the fugacity in the Final slate to that in the initial state tor steam undergoing the isothermal change of stale: (a) From 9.000 kPu and 400C to 300...
-
The number of hours of daylight that occur at any location on Earth depends on the time of year and the latitude of the location. The equations below model the numbers of hours of daylight in Seward,...
-
Bobby is a baseball player who earns $1 million a year playing for team X. If he werent playing baseball for team X, he would be playing baseball for team Y and earning $800,000 per year. If he...
-
Multiple Choice Questions Identify the best answer for each of the following 1. Which of the following statements concerning the accounting and financial reporting for capital assets is false? a....
-
How does the decision to use debt involve a risk-versus-return trade-off? AppendixLO1
-
Applying the net present value approach with and without tax considerations Paxton Kingsley, the chief executive officer of Kingsley Corporation, has assembled his top advisers to evaluate an...
-
a) Define integrated reporting. (3 marks) (CLO3:PLO6:01) b) Differentiate between integrated reporting and traditional reporting. Provide at least FOUR (4) differences. (8 marks) (CLO3:PL06:C1) c)...
-
Laura Moore has recently left her job as a Graphic Designer to open her own Company; a Graphic Design Agency dedicated to the creation and design of apps for mobile devices. Laura has decided to be...
-
Rationalize the following rule of thumb, appropriate for an equimolar binary liquid mixture: GE (equimolar) RT 1
-
An unusual type of low-pressure VLE behavior is that of double azeotropy, in which the dew and bubble curves are S-shaped, thus yielding at different compositions both a minimum-pressure and a...
-
Solve the linear programming problem by applying the simplex method to the dual problem. Minimize subject to C 14x + 8x + 20x3 x + x + 3x3 = 6 2x + x + x3 9 X, X, X3 = 0 =
-
Consider a piston with an orifice in a cylinder filled with a fluid of viscosity \(\mu\) as shown in Fig. 1.106. As the piston moves in the cylinder, the fluid flows through the orifice, giving rise...
-
Add a function to SmallWorld that computes the global clustering coefficient of a graph. The global clustering coefficient is the conditional probability that two random vertices that are neighbors...
-
Show that the generators of the algebra (33.8) are related by parity. For a Dirac wavefunction the action of parity is $P \psi(\boldsymbol{x}, t) P^{-1}=\gamma_{0} \psi(-\boldsymbol{x}, t)$, up to a...
-
Extend the algorithm you designed for Exercise 6.2 so that it can evaluate positions that are nonterminalin other words, positions where the game has not yet finished. Your score should be positive...
-
In addition to tanh, another s-shaped smooth function, the logistic sigmoid function y=1 / (1+exp(x)), is commonly used as an activation function in neural networks. A common way to implement them in...
-
The following data were taken from the income statements of Mydorf Company. Compute for each year (a) the inventory turnover and (b) days in inventory. What conclusions concerning the management of...
-
For the next several days, take notes on your listening performance during at least a half-dozen situations in class, during social activities, and at work, if applicable. Referring to the traits of...
-
Suppose ethane was compressed adiabatically in a 70% efficient continuous compressor. The downstream pressure is specified to be 1500 psia at a temperature not to exceed 350F. What is the highest...
-
Derive the integrated formula for the Helmholtz energy departure for the virial equation (Eqn. 7.7), where B is dependent on temperature only. Express your answer in terms of B and its temperature...
-
An ordinary vapor-compression cycle is to be designed for superconductor application using N2 as refrigerant. The expansion is to 1 bar. A heat sink is available at 105 K. A 5 K approach should be...
-
Case Products manufactures two models of DVD storage cases: regular and deluxe. Presented is standard cost information for each model: Cost Components Regular Deluxe Direct materials Lumber 2 board...
-
A corporate bond that you own at the beginning of the year is worth $930. During the year, it pays $56 in interest payments and ends the year valued at $920. What was your dollar return and percent...
-
Anissa makes custom bird houses in her garage and she buys all her supplies from a local lumber yard. Last year she purchased $4500 worth of supplies and produced 2500 bird houses. She sold all 2500...

Study smarter with the SolutionInn App


