For one of the substances in Table 3.2, compute the final pressure when the substance is heated
Question:
For one of the substances in Table 3.2, compute the final pressure when the substance is heated from 15°C and 1 bar to 25°C at constant volume.
Table 3.2
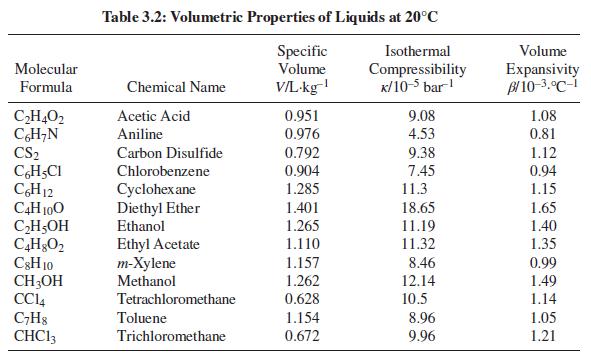
Transcribed Image Text:
Table 3.2: Volumetric Properties of Liquids at 20°C Specific Volume Isothermal Volume Molecular V/L-kg Compressibility K/10-5 bar! Expansivity Bl 10-3.°C-i Formula Chemical Name 1.08 CH,O2 CH;N CS2 Acetic Acid 0.951 0.976 9.08 Aniline 4.53 0.81 0.792 9.38 1.12 0.94 Carbon Disulfide CH,CI CH12 C4H 100 CH5OH Chlorobenzene 0.904 7.45 11.3 Cyclohex ane Diethyl Ether Ethanol 1.285 1.15 1.401 18.65 1.65 1.265 11.19 1.40 Ethyl Acetate т-Хylene Methanol 1.110 11.32 1.35 C3H 10 CH;OH CCI4 C;H8 CHC13 1.157 8.46 0.99 1.262 0.628 12.14 1.49 Tetrachloromethane 10.5 1.14 Toluene 1.154 8.96 1.05 Trichloromethane 0.672 9.96 1.21
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
To solve this problem we will use the ideal gas law PV nRT where P is the pressure V is the volume n ...View the full answer

Answered By

FELIX NYAMBWOGI
I have been tutoring for over 5 years, both in person and online. I have experience tutoring a wide range of subjects, including math, science, English, and history. I have also worked with students of all ages, from elementary school to high school.
In addition, I have received training in effective tutoring strategies and techniques, such as active listening, questioning, and feedback. I am also proficient in using online tutoring platforms, such as Zoom and Google Classroom, to effectively deliver virtual lessons.
Overall, my hands-on experience and proficiency as a tutor has allowed me to effectively support and guide students in achieving their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
For one of the substances in Table 3.2, compute the change in volume and work done when one kilogram of the substance is compressed from 1 bar to 100 bar at a constant temperature of 20C. Table 3.2...
-
For one of the substances in Table 3.2, compute the change in volume and work done when one kilogram of the substance is heated from 15C to 25C at a constant pressure of 1 bar. Table 3.2 Table 3.2:...
-
The following standard costs were developed for one of the products of ABC Company: STANDARD COST CARD PER UNIT Direct materials: 20 pounds x $10 per pound .................................. $200.00...
-
Calculate the weighted average cost of capital for Genedak-Hogan for before and after international diversification. Did the reduction in debt costs reduce the firm's weighted average cost of...
-
1. How is New Belgium Brewing Companys approach to social responsibility different from that of other companies? What are the advantages and disadvantages of this approach? New Belgium is a small,...
-
Describe what is meant when an auditor is associated with a set of financial statements.
-
Using the profit model developed in Chapter 11, implement a financial simulation model for a new product proposal and determine a distribution of profits using the discrete distributions below for...
-
High blood pressure results from constriction of the arteries. To maintain a normal flow rate (flux), the heart has to pump harder, thus increasing the blood pressure. Use Poiseuilles Law to show...
-
31 An agreement regarding a position in a specified currency, a specified exchange rate, and a specified future settlement date. Payment is made by one party to the other based on the exchange rate...
-
Zippy Cola is studying the effect of its last advertising campaign. People chosen at random were called and asked how many cans of zippy cola they hand bought and advertisements they had either read...
-
a. A vacant lot acquired for $115,000 is sold for $298,000 in cash. What is the effect of the sale on the total amount of the sellers (1) assets, (2) liabilities, and (3) owners equity? b. Assume...
-
A substance for which is a constant undergoes an isothermal, mechanically reversible process from initial state (P 1 , V 1 ) to final state (P 2 , V 2 ), where V is molar volume. (a) Starting with...
-
What amounts are included in gross income for the following taxpayers? Explain your answers. a. Janus sued Tiny Toys for personal injuries from swallowing a toy. Janus was paid $30,000 for medical...
-
Brian is considering increasing the length of the cryptographic keys used by his organization. If he adds 8 bits to the encryption key, how many more possible keys will be added to the key space for...
-
Business law SECHON A [100 Marks] Read the scenario below then answer the questions that follow. Contracts are of critical importance especially in daily commercial and business transactions....
-
You may assume that the production costs to the winery are the same for each of the possible wines, despite the differences in volumes with some of the possible wines. Thus maximizing revenue will be...
-
You encounter a split system that uses R-22 refrigerant and observe the following refrigeration parameters from the unit's control display. The unit is operating in cooling mode. Suction pressure:...
-
A refrigerant at -20C is flowing through a 4" schedule 40 carbon steel pipe (inner diameter 102 mm, outer diameter 114 mm); the heat transfer coefficient for the refrigerant is 2500 W/m/K. It is...
-
Landlord Company and Tenant Company enter into a noncancelable, direct financing lease on January 1, 2019, for nonspecialized equipment that cost the Landlord $280,000 (useful life is 6 years with no...
-
The time to assemble the first unit on a production line is 10 hours. The learning rate is 0.94. Approximately how long will it take for the seventh unit to be assembled? The number of hours needed...
-
For the acetone (1)/methanol(2) system a vapor mixture for which z1 = 0,25 and z2 = 0.75 is cooled to temperature T in the two-phase region and flow* into a separation chamber at a pressure of 1 bar....
-
A process stream contains light species 1 and heavy species 2. A relatively pure liquid stream containing mostly 2 is desired, obtained by a single-stage liquid/vapor separation. Specifications on...
-
Assuming the validity of the De Priester charts, make the following VLE calculations for the methane(1)/cthylenc(2)/elhand(3) system: (a) BVBL P. given x1 = 0.10. x2 = 0.50, and t = -60(F). (b) DEW...
-
Required : a- outline the statement of comperhensive income for the year ended 30 november 2021 b- outline the statment of financial position as at 30 November The Trial Balance of Alim Enterprise at...
-
International business and environment The MIR requires teams to gather current, or the most recently available, data on the markets people, economy, government, and technological status from online...
-
Consider the following stream of cash flows. The interest rate is 10%. 0 1 2 3 4 5 6 7 100 100 100 200 0 300 300 300 a) What is the value at time 0 of the cash flow stream? b) What is the value of...

Study smarter with the SolutionInn App


