For one of the substances in Table 3.2, compute the change in volume and work done when
Question:
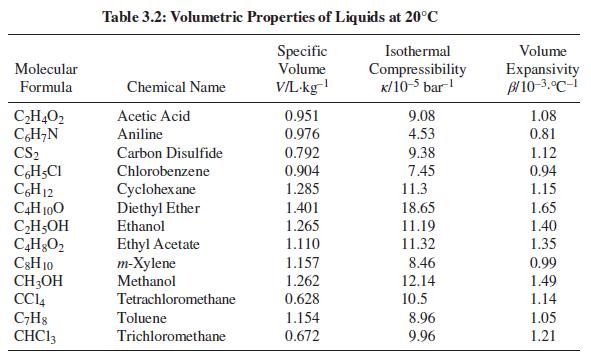
For one of the substances in Table 3.2, compute the change in volume and work done when one kilogram of the substance is heated from 15°C to 25°C at a constant pressure of 1 bar.
Table 3.2
Transcribed Image Text:
Table 3.2: Volumetric Properties of Liquids at 20°C Specific Volume Isothermal Volume Molecular V/L-kg Compressibility K/10-5 bar! Expansivity Bl 10-3.°C-i Formula Chemical Name 1.08 CH,O2 CH;N CS2 Acetic Acid 0.951 0.976 9.08 Aniline 4.53 0.81 0.792 9.38 1.12 0.94 Carbon Disulfide CH,CI CH12 C4H 100 CH5OH Chlorobenzene 0.904 7.45 11.3 Cyclohex ane Diethyl Ether Ethanol 1.285 1.15 1.401 18.65 1.65 1.265 11.19 1.40 Ethyl Acetate т-Хylene Methanol 1.110 11.32 1.35 C3H 10 CH;OH CCI4 C;H8 CHC13 1.157 8.46 0.99 1.262 0.628 12.14 1.49 Tetrachloromethane 10.5 1.14 Toluene 1.154 8.96 1.05 Trichloromethane 0.672 9.96 1.21
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)
To calculate the change in volume and work done for heating one kilogram of the substance from 15C t...View the full answer

Answered By

FELIX NYAMBWOGI
I have been tutoring for over 5 years, both in person and online. I have experience tutoring a wide range of subjects, including math, science, English, and history. I have also worked with students of all ages, from elementary school to high school.
In addition, I have received training in effective tutoring strategies and techniques, such as active listening, questioning, and feedback. I am also proficient in using online tutoring platforms, such as Zoom and Google Classroom, to effectively deliver virtual lessons.
Overall, my hands-on experience and proficiency as a tutor has allowed me to effectively support and guide students in achieving their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
For one of the substances in Table 3.2, compute the final pressure when the substance is heated from 15C and 1 bar to 25C at constant volume. Table 3.2 Table 3.2: Volumetric Properties of Liquids at...
-
A piston/cylinder device keeping a constant pressure has 1 kg water at 20oC and 1 kg of water at 100oC both at 500 kPa separated by a thin membrane. The membrane is broken and the water comes to a...
-
A gas is compressed at a constant pressure of 0.800 atm from 9.00 L to 2.00 L. In the process, 400 J of energy leaves the gas by heat. (a) What is the work done on the gas? (b) What is the change in...
-
Models that pertain to the distribution of a resource within supply chains are often referred to as networks. Distribution among these networks is key to the success of a business while also keeping...
-
What is the difference between a programmed and non-programmed decision? Consider a programmed decision and a non-programmed decision you have made recently. Compare and contrast the approaches you...
-
The CPA firm of Lumley & Lu uses a quantitative approach to implementing the audit risk model. Calculate detection risk for each of the following hypo thetical clients. Risk of Material Misstatement...
-
A long-standing topic in the social sciences is the authoritarian personality: there are certain characteristics that lead people to favor a very strong government. One of these factors is trust. Is...
-
Preparing a classified balance sheet Required Use the following information to prepare a classified balance sheet for Steller Co. at the end of 2012. Accounts receivable .... $42,500 Accounts payable...
-
19. On January 1, 2018, Robinson Company purchased Franklin Company at a price of $3,820,000. The fair market value of the net assets purchased equals $2,760,000. What is the amount of goodwill that...
-
Genesis Ltd was incorporated three years ago and has grown rapidly since then. The rapid rate of growth has created problems for the business, which the directors have found difficult to deal with....
-
For one of the substances in Table 3.2, compute the change in volume and work done when one kilogram of the substance is compressed from 1 bar to 100 bar at a constant temperature of 20C. Table 3.2...
-
Various species of hagfish, or slime eels, live on the ocean floor, where they burrow inside other fish, eating them from the inside out and secreting copious amounts of slime. Their skins are widely...
-
The marginal cost of educating a college student online is $3,000 a year. The table shows the marginal private benefit schedule from a college education. The marginal external benefit is 50 percent...
-
Pacifico Company, a U . S . - based importer of beer and wine, purchased 1 , 7 0 0 cases of Oktoberfest - style beer from a German supplier for 4 5 9 , 0 0 0 euros. Relevant U . S . dollar exchange...
-
Consider each of the following scenarios and identify a behavioral intervention to address each issue in family work. A teenager not complying with curfew. One member of the couple not picking up...
-
Sandy Crane Hospital expanded its maternity ward to add patient rooms for extended hospital stays. They negotiated a 15-year loan with monthly payments and a large sum of $250,000 due at the end of...
-
2 (39 marks) R QUESTION 2 (39 marks) Roundworm Ltd is a group of companies with a 31 December year-end. The Roundworm group financial statements for the years 20.21 and 20.22 are given below:...
-
Vino Veritas Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,400 cases of wine at a price of 240 euros per case. The total purchase price is 336,000...
-
On October 4, 2019, Collins Company purchased 100 bonds of Steph Company for $6,400 as a short-term investment in securities classified as available for sale. On December 31, 2019, the bonds had a...
-
Wal-Mart is the second largest retailer in the world. The data file on the disk holds monthly data on Wal-Marts revenue, along with several possibly related economic variables. a) Using computer...
-
Steam at 2,000 kPa containing 6% moisture is heated at constant pressure to 575oC. How much heat is required per kilogram?
-
Steam at 2.700 kPa and with a quality of 0.90 undergoes a reversible, adiabatic expansion in a non-flow process to 400 kPa. It is then healed at constant volume until it is saturated vapor. Determine...
-
Four kilograms of steam in a piston/cytinder device at 400 kPa and 175oC undergoes a mechanically reversible, isothermal compression to a final pressure such that the steam is just saturated....
-
Your firm is planning to invest in an automated packaging plant. Harburtin Industries is an all - equity firm that specializes in this business. Suppose Harburtin ' s equity beta is 0 . 8 7 , the...
-
Ned Allen opened a medical practice in Los Angeles, California, and had the following transactions during the month of January. (Click the icon to view the January transactions.) Journalize the...
-
do you need more information or are you working on this? Irene Watts and John Lyon are forming a partnership to which Watts will devote one- half time and Lyon will devote full time. They have...

Study smarter with the SolutionInn App


