For the ammonia synthesis reaction written: With 0.5 mol N 2 and 1.5 mol H 2 as
Question:
For the ammonia synthesis reaction written:
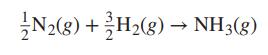
With 0.5 mol N2 and 1.5 mol H2 as the initial amounts of reactants and with the assumption that the equilibrium mixture is in the ideal gas state, show that:
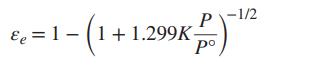
Transcribed Image Text:
N2(8) + H₂(g) → NH3(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
It seems like you want to analyze the ammonia synthesis reaction and its equilibrium using the given ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781260597684
9th International Edition
Authors: J.M. Smith, Mark Swihart Hendrick C. Van Ness, Michael Abbott
Question Posted:
Students also viewed these Engineering questions
-
For the ammonia synthesis reaction written: with 0.5 mol N 2 and 1.5 mol H 2 as the initial amounts of reactants and with the assumption that the equilibrium mixture is an ideal gas, show that: N2{g)...
-
During an experiment with the Haber process, a researcher put 1 mol N2 and 1 mol H2 into a reaction vessel to observe the equilibrium formation of ammonia, NH3. When these reactants come to...
-
Air flows with negligible friction through a 4-indiameter duct at a rate of = lbm/s. The temperature and pressure at the inlet are T1 = 800 R and P1 = 30 psia, and the Mach number at the exit is Ma2...
-
The concept of beta is most closely associated with: a. Correlation coefficients. b. Mean-variance analysis. c. Nonsystematic risk. d. Systematic risk.
-
Can there be a difference in the gross margin percentages of individual joint products resulting from allocation of joint costs on the basis of physical quantities? Sales values? LO.1
-
A company paid cash dividends of $0.81 per share. Its earnings per share is $6.95 and its market price per share is $45.00. Its dividend yield is: a. 1.8% b. 11.7% c. 15.4% d. 55.6% e. 8.6%...
-
You are analyzing various programs to reduce water pollution from food processing plants. In consultation with your staff, you have developed the following matrix of effects (where PV = Present...
-
The Hawaii Department of Transportation built a new bridge over the Mississippi River for a cost of $150.00 million. It is estimated that the cost to maintain the bridge will be $1,626,599 per year...
-
In the Reddy Mikks model of Example 2.2-1; (a) Determine the range for the ratio of the unit revenue of exterior paint to the unit revenue of interior paint. (b) If the revenue per ton of exterior...
-
Carbon black is produced by the decomposition of methane: For equilibrium at 650C and 1 bar, (a). What is the gas-phase composition if pure methane enters the reactor, and what fraction of the...
-
A system formed initially of 2 mol CO 2 , 5 mol H 2 , and 1 mol CO undergoes the reactions: Develop expressions for the mole fractions of the reacting species as functions of the reaction coordinates...
-
Explain why succinyl-CoA arising from the oxidation of odd-chain fatty acids cannot be directly oxidized by the citric acid cycle. How can it be further degraded?
-
The process of translating an idea into goods and services that create value or for which clients will pay is called
-
Let f be twice differentiable with f(0) = 6, f(1) = 8, and f'(1) = 7. Evaluate the following integral. [ = 0 0 xf" (x)dx
-
Although the Chen Company's milling machine is old, it is still in relatively good working order and would last for another 10 years. It is inefficient compared to modern standards, though, and so...
-
PART-3: OFFLINE QUESTIONS - Upload files using the submission link. 1. In 2020 Starbucks began a secret project to develop a competing product against the Keurig Single Serve coffee brewer. The...
-
As a leader, what are your highest values? o What's the contribution you want to make as a leader o What makes you distinct as a leader? o Drawing from StrengthsFinder 2.0 what are your strengths as...
-
Hallergan Company produces car and truck batteries that it sells primarily to auto manufacturers. Dorothy Hawkins, the company's controller, is preparing the financial statements for the year ended...
-
Akramin just graduated with a Master of Engineering in Manufacturing Engineering and landed a new job in Melaka with a starting salary of RM 4,000 per month. There are a number of things that he...
-
Use the UNIFAC model to predict P-x-y data at 90C and x 1 , = {0, 0.1, 0.3, 0.5, 0.7, 0.9, 1.0} for propanoic acid + water. Fit the UNIQUAC model to the predicted P-x-y data and report your UNIQUAC a...
-
(a) Rearrange Eqn. 13.22 to obtain Eqn. 13.23. (b) Use Eqns. 13.16 and 13.18 in Eqn. 13.17 and perform the integration to obtain Eqn. 13.19. (c) Use Eqns. 13.16 and 13.31 in Eqn. 13.17 and perform...
-
The energy equation for mixtures can be written for polymers in the form: By analogy to the development of the Scatchard-Hildebrand theory, this can be rearranged to: where N di = degree of...
-
Which of the following concerning short-term financing methods is NOT CORRECT? Short-term bank loans typically do not require assets as collateral. Firms generally have little control over the level...
-
Kingbird Corporation is preparing its December 31, 2017, balance sheet. The following items may be reported as either a current or long-term liability. 1. On December 15, 2017, Kingbird declared a...
-
BE13.2 (LO 1), AP An inexperienced accountant for Silva Corporation showed the following in the income statement: net income \$337,500 and unrealized gain on availablefor-sale securities (before...

Study smarter with the SolutionInn App


