Given below are four proposed modifications of the van der Waals equation of state. Are any of
Question:
Given below are four proposed modifications of the van der Waals equation of state. Are any of these modifications reasonable? Explain carefully; statements such as, “It isn’t cubic in volume” do not qualify.
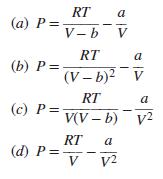
Transcribed Image Text:
RT (а) Р%3 a V- b V RT a (b) P = (V – b)? V RT a (c) P= V(V – b) v2 RT (d) P = V a V2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
The van der Waals equation of state is For carbon dioxide, a = 0.3640 Pa m 6 mol 1 and b = 4.267 Ã 10 5 m 3 mol 1 . Find the pressure of 0.7500 mol of carbon dioxide if V = 0.0242 m 3 and T =...
-
The van der Waals equation of state (Equation 5.3-6) is to be used to estimate the specific molar volume V (L/mol) of air at specified values of T (K) and P(atm). The van der Waals constants for air...
-
The van der Waals equation of state (Equation 5.3-7) is to be used to estimate the specific molar volume V(L/mol) of air at specified values of T(K) and P(atm). The van der Waals constants for air...
-
The National Health Statistics Reports dated Oct. 22, 2008, included the following information on the heights (in.) for non-Hispanic white females: a. Calculate and interpret a confidence interval at...
-
Is a firm justified in paying a bribe if it believes a competitor will do so to win an important contract? Explain.
-
How many years (and months) will it take $2 million to grow to $5 million with an annual interest rate of 7 percent?
-
Define and use custom distributions in Monte Carlo simulations.
-
This exercise continues the Lawlor Lawn Service, Inc., situation from Exercise 4-36 of Chapter 4. Lawlor Lawn Service has also begun selling plants that it purchases from a wholesaler. During June,...
-
Mirabile Corporation uses activity-based costing to compute product margins. Overhead costs have already been allocated to the company's three activity cost pools--Processing, Supervising, and Other....
-
Zippy Cola is studying the effect of its last advertising campaign. People chosen at random were called and asked how many cans of zippy cola they hand bought and advertisements they had either read...
-
With reference to Prob. 2.47, assume air to be an ideal gas, and develop an expression giving the household air temperature as a function of time. Problem 2.47 The heating of a home to increase its...
-
For a steady-flow heat exchanger with a feed temperature of 100C, compute the outlet stream temperature when heat in the amount of 12 kJmol 1 is added to the following substances. (a) Methane (b)...
-
Refer to the Centers for Disease Control and Prevention listing of the October 2015 sanitation scores for 195 cruise ships, Exercise 2.24. a. Find the mean and standard deviation of the sanitation...
-
Assume that John wants to annuitize the annuity and is told that he can receive a straight life annuity for $600 a month for life. If the actuarial number of payments is 300, how much of the first...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 90% confident that the estimated percentage is in error...
-
Your homework for this week is to watch the first lecture on Financial Accounting and at the end of the outline there are several problems for you to do. The problems begin with parts A-D for you to...
-
Sheril Rose was a brilliant but penniless material scientist. She had designed a new type of solar panel she believed had great commercial potential. On January 15, she approached Felda Higgins, a...
-
IAS 23 requires companies to capitalize borrowing costs directly attributable to the acquisition, construction or production of an asset into the cost of an asset.Previously, accounting standard...
-
Rexon Company leases non-specialized equipment to Ten-Care Company beginning January 1, 2019. The lease terms, provisions, and related events are as follows: 1. The lease term is 8 years. The lease...
-
On 1 July 2021, Croydon Ltd leased ten excavators for five years from Machines4U Ltd. The excavators are expected to have an economic life of 6 years, after which time they will have an expected...
-
Calculate the theoretical flame temperature when ethylene at 25(C is burned with: (a) The theoretical amount of air at 25(C. (b) 25% excess air at 25(C. (c) 50% excess air at 25(C. (d) 100% excess...
-
For steady Bow through a heat exchanger at approximately atmospheric pressure, what is the final temperature, (a) When heat in the amount of 800 kJ is added to 10 mol of ethylene initially at 200(C?...
-
For steady Bow through a heat exchanger at approximately atmospheric pressure, what is the final temperature, (a) When heat in the amount of 800 kJ is added to 10 mol of ethylene initially at 200(C?...
-
An 8%, 30-year semi-annual corporate bond was recently being priced to yield 10%. The Macaulay duration for this bond is 10.2 years. What is the bonds modified duration? How much will the price of...
-
Question 7 of 7 0/14 W PIERDERY Current Attempt in Progress Your answer is incorrect Buffalo Corporation adopted the dollar value LIFO retail inventory method on January 1, 2019. At that time the...
-
Cost of debt with fees . Kenny Enterprises will issue a bond with a par value of $1,000, a maturity of twenty years, and a coupon rate of 9.9% with semiannual payments, and will use an investment...

Study smarter with the SolutionInn App


