Prove that For an ideal gas with constant heat capacities, use this result to derive Eq. (3.23c).
Question:
Prove that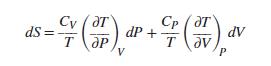 For an ideal gas with constant heat capacities, use this result to derive Eq. (3.23c).
For an ideal gas with constant heat capacities, use this result to derive Eq. (3.23c).
Eq. (3.23c)
![]()
Transcribed Image Text:
Cy ( aT Cp ( aT ds = dP + dV T av P V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (15 reviews)
Starting with the definition of entropy ds as the differential of enthalpy dH minus the product of t...View the full answer

Answered By

Dulal Roy
As a tutor, I have gained extensive hands-on experience working with students one-on-one and in small group settings. I have developed the ability to effectively assess my students' strengths and weaknesses, and to customize my teaching approach to meet their individual needs.
I am proficient at breaking down complex concepts into simpler, more digestible pieces, and at using a variety of teaching methods (such as visual aids, examples, and interactive exercises) to engage my students and help them understand and retain the material.
I have also gained a lot of experience in providing feedback and guidance to my students, helping them to develop their problem-solving skills and to become more independent learners. Overall, my hands-on experience as a tutor has given me a deep understanding of how to effectively support and encourage students in their learning journey.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
For an ideal gas with constant heat capacities, show that: (a) For a temperature change from T 1 to T 2 , S of the gas is greater when the change occurs at constant pressure than when it occurs at...
-
An ideal gas with the adiabatic exponent undergoes a process in which its internal energy relates to the volume as U = aV, where a and a are constants. Find: (a) The work performed by the gas and the...
-
An ideal gas with the adiabatic exponent y goes through a process p = Po- aV, where Po and a are positive constants, and V is the volume. At what volume will the gas entropy have the maximum value?
-
The Trial Balance and Adjustments columns of the worksheet of Wells Decorating Centre included these accounts and balances at December 31, 2017: Required Wells Decorating Centre uses the perpetual...
-
Explain four listening principles that are discussed in your text.
-
What would you expect to happen to investment and growth in the economy if the U.S. government decided to abolish the Securities and Exchange Commission?
-
If you were to follow up the Slonaker and Wendt (2003) study on discrimination against African American males, what philosophical stance may underpin your research choice? LO6
-
Magnificent Modems, Inc., acquired a subsidiary named Anywhere, Inc. (AI). AI manufactures a wireless modem that enables users to access the Internet through cell phones. The following trial balance...
-
Please help with number 3 and be very specific.. I need to understand where the numbers are coming from. Those who have answered this question for others on here have not been specific and have just...
-
Assume that the Provident Health System, a for-profit hospital, has $1 million in taxable income for 2008, and its tax rate is 30 percent. A. Given this information, what is the firm's net income? B....
-
A tank of 4 m 3 capacity contains 1500 kg of liquid water at 250C in equilibrium with its vapor, which fills the rest of the tank. A quantity of 1000 kg of water at 50C is pumped into the tank. How...
-
Propane gas at 100C is compressed isothermally from an initial pressure of 1 bar to a final pressure of 10 bar. Estimate H and S.
-
YiLing, the sole shareholder of Brown Corporation, sold her Brown stock to Calvin on July 30 for $270,000. YiLings basis in the stock was $200,000 at the beginning of the year. Brown had accumulated...
-
Use the Comprehensive Annual Financial Report for the Village of Arlington Heights (please look up this content) for the year ended December 31, 2018, to answer questions 8-20. All questions are on...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Two wires lie perpendicular to the plane of the screen and carry equal magnitudes of electric current in the directions shown. Point P is equidistant from the two wires. The distance between each of...
-
Your firm has recently been appointed as auditors of Kentronics Ltd , a large company which markets sophisticated electronic equipment for heavy industry as well as the mining equipment industry. The...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Bailey Company has had a defined benefit pension plan for several years. At the end of 2019, Baileys actuary provided the following information for 2019 regarding the pension plan: (1) service cost,...
-
Dr. Chan obtained a $15,000 demand loan at prime plus 1.5% on September 13 from the Bank of Montreal to purchase a new dental X-ray machine. Fixed payments of $700 will be deducted from the dentists...
-
Estimate the molar volume, enthalpy, and entropy for n-butane as a saturated vapor and as a saturated liquid at 370 K. The enthalpy and entropy are set equal to zero for the ideal-gas state at 101,...
-
Estimate the molar volume, enthalpy, and entropy for n-butane as a saturated vapor and as a saturated liquid at 370 K. The enthalpy and entropy are set equal to zero for the ideal-gas state at 101,...
-
The total steam demand of a plant over the period of an hour is 6,000 kg, but instantaneous demand fluctuates from 4.000 to 10.000 kg hr-1. Steady boiler operation at 6.000 kg hr~1 is accommodated by...
-
Diplomatic Security Service provides Airport Transportation and Surveillance Service to Foreign Diplomats in Guyana. The company has two support departments - Information Systems and Equipment...
-
Q1: A disparity of bargaining power between the parties to a contract may result in unfair terms but a court is not likely to consider the contract unconscionable. Group of answer choices a. True b....
-
Life Tool Manufacturing has a system in place to recall products that prove to be dangerous at some time after manufacture and distribution. This represents which element of the due care theory?...

Study smarter with the SolutionInn App


