Recent research suggests the following equation of state, known as the PC-SAFT model. (a) Derive an expression
Question:
Recent research suggests the following equation of state, known as the PC-SAFT model.
(a) Derive an expression for Z.
(b) Derive the departure function for (U-Uig).
ηP = b; m = constant proportional to molecular weight; ai, bi are constants.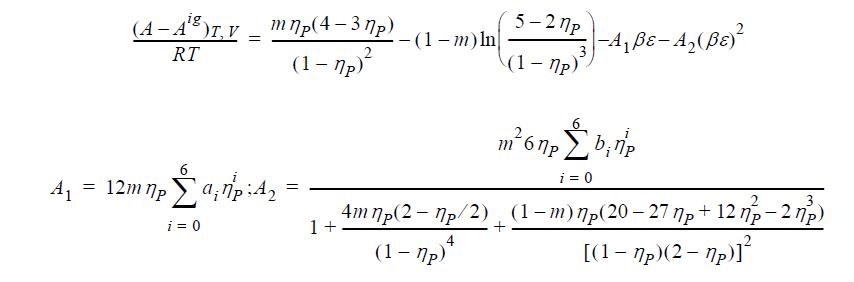
Transcribed Image Text:
(A - A°)T, V mnp(4-3np) ig. = RT 2 (1-7p)² = A₁ 12m np a₁7:4₂ i = 0 = -(1-m) ln 5-27p (1-7p) -A₁ßε-A₂ (ßε)² m²6 npb₁np i = 0 4 m np(2-7p/2) (1-m) mp(20-27 p + 12 np - 27p) 1+ (1-7p)* [(1-7p)(2-7p)]²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:
Students also viewed these Engineering questions
-
Derive an expression for the speed of sound based on van der Waals equation of state P = RT(v - b) - a/v2. Using this relation, determine the speed of sound in carbon dioxide at 50C and 200 kPa, and...
-
Derive an expression for the speed of sound based on van der Waals' equation of state P = RT(v - b) - a/v2. Using this relation, determine the speed of sound in carbon dioxide at 808C and 320 kPa,...
-
Recent research in thermodynamic perturbation theory suggests the following equation of state. (a) Derive the departure function for (A A ig ) T,V . (b) Derive the departure function for (U U ig )....
-
Using the 526 observations on workers in a certain dataset, we include educ (years of education), exper (years of labor market experience) and tenure (years with the current employer) in regression...
-
Draw the marginal-cost and average-total-cost curves for a typical firm. Explain why the curves have the shapes that they do and why they cross where they do.
-
What are the strengths of Expectancy/valence Theory? Apply this theory to a concrete example at work.
-
What is co-branding?
-
Oregon Forests uses a joint process to manufacture two grades of wood: A and B. During October 2013, the company incurred $ 16,200,000 of joint production cost in producing 27,000,000 board feet of...
-
consider a situation where homebuyers find of their dream priced at 330,000 they saved a 20% down payment and are pre-qualified for a 10 year 264,000 fixed rate mortgage at 6.5%. Recalling that...
-
P-V-T behavior of a simple fluid is found to obey the equation of state given in problem 8.14. (a) Derive a formula for the enthalpy departure for the fluid. (b) Determine the enthalpy departure at...
-
Suppose ethane was compressed adiabatically in a 70% efficient continuous compressor. The downstream pressure is specified to be 1500 psia at a temperature not to exceed 350F. What is the highest...
-
Identify the solid
-
Scenario : Wanda, a BCBA, is updating an intervention plan for a leaner on her caseload to submit for insurance funding authorization. Part of the plan includes the completion of an adaptive...
-
a) how can your company accommodate generational or gender difference within your company ? b) how can your company accommodate communication and or language difference within your company ?
-
3. Consider the following data for two catalysts, A and B. The temperature is 25 C and the reaction occurs at standard conditions. a. Make a Tafel plot and determine the Tafel slope. Estimate the...
-
How consumption can be helpful in facilitating the construction of your identity? Explain the ways in which the symbolic meanings, connected with your consumption choices are important to you? Is...
-
Cataumet Boats, Inc. Jaime Giancola had just completed the first half of her MBA program and wanted to work on a project during the summer that would give her some practical experience applying what...
-
Ladd Corporation sells construction equipment to a customer for $200,000. The equipment comes with a standard 10-year warranty covering all maintenance and repairs during that time. Initially, Ladd...
-
(a) Given a mean free path = 0.4 nm and a mean speed vav = 1.17 105 m/s for the current flow in copper at a temperature of 300 K, calculate the classical value for the resistivity of copper. (b)...
-
What is the heat effect when 20 kg of LiCl(s) is added to 125 kg of an aqueous solution containing 10-wt-% LiCl in an isothermal process at 25C?
-
If a liquid solution of HCl in water, containing 1 mol of HCl and 4.5 mol of H 2 O, absorbs an additional 1 mol of HCl(g) at a constant temperature of 25C, what is the heat effect?
-
If LiCl2H 2 O(s) and H 2 O(l) are mixed isothermally at 25C to form a solution containing 10 mol of water for each mole of LiCl, what is the heat effect per mole of solution?
-
En prenant un exemple de votre choix, montrer comment on value un swap de taux de change.
-
How much would you need to invest today in order to receive: a. $10,000 in 5 years at 11%? b. $11,000 in 12 years at 8%? c. $12,000 each year for 10 years at 8%? d. $12,000 at the beginning of each...
-
A company that manufactures pulse Doppler insertion flow meters uses the Straight Line method for book depreciation purposes. Newly acquired equipment has a first cost of $190,000 with a 3-year life...

Study smarter with the SolutionInn App


