Refer to Example 2.10 about transformation of kinetic energy to enthalpy. Instead of water, suppose N 2
Question:
Refer to Example 2.10 about transformation of kinetic energy to enthalpy. Instead of water, suppose N2 at 1 bar and 298 K was flowing in the pipe. How would that change the answers? In particular, how would the temperature rise change?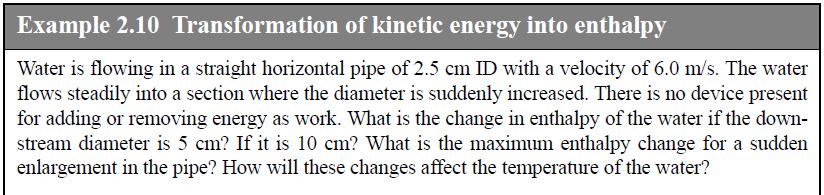
Transcribed Image Text:
Example 2.10 Transformation of kinetic energy into enthalpy Water is flowing in a straight horizontal pipe of 2.5 cm ID with a velocity of 6.0 m/s. The water flows steadily into a section where the diameter is suddenly increased. There is no device present for adding or removing energy as work. What is the change in enthalpy of the water if the down- stream diameter is 5 cm? If it is 10 cm? What is the maximum enthalpy change for a sudden enlargement in the pipe? How will these changes affect the temperature of the water?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Change in enthalpy of N2 The change in enthalpy of N2 can be calculated using the following equation ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:
Students also viewed these Engineering questions
-
Two moles of nitrogen are initially at 10 bar and 600 K (state 1) in a horizontal piston/cylinder device. They are expanded adiabatically to 1 bar (state 2). They are then heated at constant volume...
-
a. On January 1, 20X1, Stella Entity purchases bonds issued by Gragas Entity for $900,000. Stella Entity will receive an annual coupon payment of $75,000 and an additional $1,000,000 when the bond...
-
The experimentally determined density of O 2 at 140. bar and 298 K is 192 g L 1 . Calculate z and V m from this information. Compare this result with what you would have estimated from Figure 7.8....
-
Varatta Enterprises sells industrial plumbing valves. The following table lists the annual sales amounts for a portion of the salespeople in the organization for the most recent fiscal year. Click on...
-
Use the cash flow diagram to determine the single amount of money Q4 in year 4 that is equivalent to all of the cash flows shown. Use i = 10% peryear. |-10% per year 101 23 5 6 78 Year $25 $25 $25...
-
How do these preoccupations differ from those of quantitative researchers, which were considered in Chapter 8?
-
Are there national accounting standards making reference to IPSAS?
-
Carl has had a couple of good years in his new business. However, in the current year he has a net operating loss of $80,000. He does not feel that the future of his business is very bright. As his...
-
Companies that have a high demand for making copies, both color and black and white, often choose to lease a high - end copier that provides fast and reliable service at a reasonable cost. The lease...
-
Argon at 400 K and 50 bar is adiabatically and reversibly expanded to 1 bar through a turbine in a steady process. Compute the outlet temperature and work derived per mole.
-
Saturated steam at 660 F is adiabatically throttled through a valve to atmospheric pressure in a steady-state flow process. Estimate the outlet quality of the steam.
-
A car initially at rest experiences loss of its parking brake and rolls down a straight hill with a constant acceleration of 0.850 m/s2 traveling a total of 100 m. Lets designate the first half of...
-
Figure < 4 ft/s 45 0.75 ft 3 ft/s 1.50 ft 1 of 1 < Part A Determine the velocity of point A on the rim of the gear at the instant shown.(Figure 1) Enter the x and y components of the velocity...
-
what ways can leaders facilitate cognitive reframing and emotional regulation techniques to promote constructive conflict resolution ?
-
What is the level of sales needed to achieve a 10% return on an investment of $10,000,000 for a restaurant (the restaurant has main products it sells: food, beverage and gift shop items) and cover...
-
1. An online computer assembling mobile phone Application provides interfaces for end users to assemble computers by selecting computer accessories with different configurations from different...
-
1. (# 3.21, Text) Plot the longitudinal and transverse coefficients of thermal expansion for a unidirectional glass-polyester composite as functions of fiber volume fraction. Assume the following...
-
Assume that the pension benefit formula of ABC Corporation calls for paying a pension benefit of $250 per year for each year of service with the company plus 50% of the projected last year's salary...
-
Identify the tax issues or problems suggested by the following situations. State each issue as a question. Jennifer did not file a tax return for 2007 because she honestly believed that no tax was...
-
Why are research and development costs expensed? Is this consistent with how other similar costs are handled? Explain why or why not. Should research and development costs be expensed? Explain why or...
-
Consider the gas-phase isomerization of cyclopropane. Are the following data of the observed rate constant as a function of pressure consistent with the Lindemann mechanism? CH, CH,CH=CH2 CH,-CH2 k...
-
Although it can be formally proved that the code in Table 10.3 is both linear and cyclic, use only two tests to partially prove the fact: Table 10.3 a. Test the cyclic property on codeword 0101100....
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


