Consider the gas-phase isomerization of cyclopropane. Are the following data of the observed rate constant as a
Question:
Are the following data of the observed rate constant as a function of pressure consistent with the Lindemann mechanism?
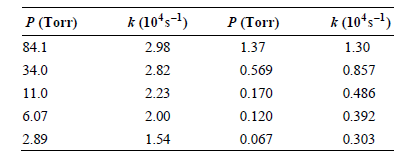
Transcribed Image Text:
CH, CH,CH=CH2 CH,-CH2 k (10ʻs-1) 2.98 2.82 P (Torr) 84.1 к (10'5-1) P (Torr) 1.37 1.30 0.569 0.170 0.857 0.486 34.0 2.23 11.0 6.07 2.00 0.120 0.392 0.067 2.89 1.54 0.303
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (17 reviews)
If the data obeyed the Lindemann mechanism then a plot of the data would fit the equ...View the full answer

Answered By

Ayush Jain
Subjects in which i am expert:
Computer Science :All subjects (Eg. Networking,Database ,Operating System,Information Security,)
Programming : C. C++, Python, Java, Machine Learning,Php
Android App Development, Xamarin, VS app development
Essay Writing
Research Paper
History, Management Subjects
Mathematics :Till Graduate Level
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The isomerization of cyclopropane, C3H6, to propylene, CH2=CHCH3, is first order in cyclopropane and first order overall. At 1000oC, the rate constant is 9.2/s. What is the half-life of cyclopropane...
-
The isomerization of cyclopropane, C3H6, is believed to occur by the mechanism shown in the following equations: Here C3H6* is an excited cyclopropane molecule. At low pressure, Step 1 is much slower...
-
From the following data for the first-order gas-phase isomerization of CH3NC at 215 oC, calculate the first-order rate constant and half-life for the reaction: Time (s) Pressure CH3NC (torr)...
-
Find the exact value of sin(x - y) if sin(x) = 3T 3T T
-
Use the graph of the function f to create a table of values for the given points. Then create a second table that can be used to find f-1, and sketch the graph of f-1, if possible. 1. 2. y 4. + 2 4...
-
The scalar and vector potentials satisfy the homogeneous wave equation in free space. Often, we choose = 0 and require that the polarization vector e of the vector potential be transverse to its...
-
Using exponential smoothing, calculate the forecasts for months 2, 3, 4, 5, and 6. The smoothing constant is 0.2, and the old forecast for month 1 is 245. Month Actual Demand Forecast Demand 1 260 2...
-
Unlike individual incentive programs, group and companywide incentive programs reward individuals based on the group and companywide performance standards, respectively. Under group and companywide...
-
20) The price at which a dealer will purchase a bond is referred to as the a) asked b) face c) call d) put e) bid
-
Explain how the product manager of a breakfast cereal might change consumer attitudes toward the companys brand by: (a) Changing beliefs about the brand; (b) Changing beliefs about competing brands;...
-
In the discussion of the Lindemann mechanism, it was assumed that the rate of activation by collision with another reactant molecule, A, was the same as collision with a nonreactant molecule, M, such...
-
Consider the following mechanism, which results in the formation of product P: If only the species A is present at t = 0, what is the expression for the concentration of P as a function of time? You...
-
Why is Valuation of Goodwill needed?
-
Problem 8-19 (Algo) Cash Budget; Income Statement; Balance Sheet [LO8-2, LO8-4, LO8-8, LO8-9, LO8- 10] Minden Company is a wholesale distributor of premium European chocolates. The company's balance...
-
Consider the unsteady flow of a fluid in the x direction through a control volume. The linear momentum of the fluid within the control volume is a function of time given by 200ti slug*ft/s, where t...
-
For a continuous uniform distribution with u = 0 and o = 1, the minimum is - V3 and the maximum is V3. For this continuous uniform distribution, find the probability of randomly selecting a value...
-
Marc Goudreau, administrator of Clearwater Hospital, was puzzled by the prior month's reports. "Every month, it's anyone's guess whether the lab will show a profit or a loss. Perhaps the only answer...
-
A system consisting of a gas consisting of O2 (32 Da), H2 (2 Da), and Ar (40 Da) molecules and a billiard ball is at some temperature . Relative to O2, the billiard ball is 1.0 E+26 times as massive...
-
In Exercises 2738, evaluate each function at the given values of the independent variable and simplify. a. b. c. f(r) = 25 r - 6 -
-
Using the parallel-axis theorem, determine the product of inertia of the area shown with respect to the centroidal x and y axes. 6 in. 9 in. 9 in- 4.5 in. in. 4.5 in.
-
An equimolar mixture of H 2 and CO is obtained by the reaction of steam with coal. The product mixture is known as water-gas. To enhance the H 2 content, steam is mixed with water-gas and passed over...
-
For the systems specified below, obtain residue curve maps and plot the range of possible distillate and bottoms compositions for the given feeds. UNIQUAC parameters are provided. (a) methanol(1) +...
-
(a) Rank the following molecules in order of increasing oxidation of carbon and give the oxidation state of C for each: CO2, -COH(aldehyde), -COOH(carboxylic acid), -CO- (ketone), -COH(alcohol),...
-
assume that we have only two following risk assets (stock 1&2) in the market. stock 1 - E(r) = 20%, std 20% stock 2- E(r) = 10%, std 20% the correlation coefficient between stock 1 and 2 is 0. and...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...

Study smarter with the SolutionInn App


