The following is a rule of thumb: For a binary system in VLE at low pressure, the
Question:
The following is a rule of thumb: For a binary system in VLE at low pressure, the equilibrium vapor-phase mole fraction y1 corresponding to an equimolar liquid mixture is approximately
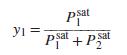
where Psati is a pure-species vapor pressure. Clearly, this equation is valid if Raoult’s law applies. Prove that it is also valid for VLE described by Eq. (13.19), with ln γ 1 = A x22 and ln γ2 = A x21.
Eq. (13.19)
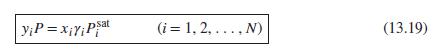
Transcribed Image Text:
Sat yi = sat sat
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
We start by writing the Kvalue which is the ratio of vaporphase to liquidphase mole fraction of comp...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
The following is a summary of all relevant transactions of Vicario Corporation since it was organized in 2012. In 2012, 15,000 shares were authorized and 7,000 shares of common stock ($50 par value)...
-
The following is a 2-way ANOVA conducted using our final project data base. Sex and Treatment Condition are your independent variables, and Post-Treatment Anxiety (RCMAS) is your dependent variable....
-
The following is a summary of all relevant transactions of Vicario Corporation since it was organized in 2014. In 2014, 15,000 shares were authorized and 7,000 shares of common stock ($50 par value)...
-
You firm needs to pay its British supplier 1,000,000. If the exchange rate is $1.61/, how many dollars will you need to pay the British supplier? OA. $1,000,000 OB. $621,118 c. $1,610,000 D. $385,787
-
It has been said that a society with a high savings rate is a society with a high standard of living. What is the link (if any) between saving and a relatively high standard of living?
-
What is the difference between resources designated by hospital boards for specific purposes and those restricted by outside donors for specific purposes? What are the differing accounting effects?
-
In year 5, the IRS audited the couples joint year 2 tax return and each spouses separate year 3 tax returns. The IRS determined that the year 2 joint return and Crewellas separate year 3 tax return...
-
Lyon Center began operations on July 1. It uses a perpetual inventory system. During July, the company had the following purchases and sales. Instructions (a) Determine the ending inventory under a...
-
1. Identify the specific instances where you feel Vince/Westchester have weaknesses in their internal control systems, and provide a potential solution to each problem. Be specific.
-
1. Was Sagarnara an intended third party beneficiary under Dr. Garcias life insurance policy giving her standing to sue the insurer? 2. Did Prudential Insurance Co. violate Dr. Garcias life insurance...
-
Flash calculations are simpler for binary systems than for the general multicomponent case because the equilibrium compositions for a binary are independent of the overall composition. Show that, for...
-
The following is a set of VLE data for the system methanol(1)/water(2) at 333.15 K: (a) Basing calculations on Eq. (13.24), find parameter values for the Margules equation that provide the best fit...
-
You are the external auditor of ZYX plc. The company has a year end of 31 December 1988. On reading the minutes of board meetings, you discovered the following: (a) On 26 june 1988, an employee was...
-
Refer to Figure 11.2: Is it more costly to build in Los Angeles or in Washington DC? What is the cost difference? Figure 11.2 Location Factors Costs shown in RSMeans Square Foot Costs are based on...
-
Suppose the prism in Figure P33.27 is immersed in a liquid in which the speed of light is lower than the speed of light in glass. Describe what happens to the light shown entering at normal...
-
Each year, the AICPA issues a general audit risk alert document and a number of industry audit risk alerts. If you can obtain access to a current copy of either the general alert or one of the...
-
The multieffect distillation system shown in Figure 11-4 appears to be able to cut energy use in half; however, the reduction is not this large. Explain why. Figure 11-4 F PL D, D Reflux B PH
-
Schemes 11-6E and 11-6F accomplish the same task of removing and purifying an intermediate component. a. What factors enter into the decision to use scheme \(11-6 \mathrm{~F}\) instead of \(11-6...
-
Data for Warner Company are presented in P12.7A. Further analysis reveals the following. 1. Accounts payable pertain to merchandise suppliers. 2. All operating expenses except for depreciation were...
-
Rowland Textile Inc. manufactures two products: sweatshirts and T-shirts. The manufacturing process involves two activities: cutting and sewing. Expected overhead costs and cost drivers are as...
-
Benzene and ethanol (e) form azeotropic mixtures. Prepare a y-x and a P-x-y diagram for the benzene-ethanol system at 45C assuming the mixture is ideal. Compare the results with the experimental data...
-
For a separations process it is necessary to determine the VLE compositions of a mixture of ethyl bromide and n-heptane at 30C. At this temperature the vapor pressure of pure ethyl bromide is 0.7569...
-
The stream from a gas well consists of 90 mol% methane, 5 mol% ethane, 3 mol% propane, and 2 mol% n-butane. This stream is flashed isothermally at 233 K and 70 bar. Use the shortcut K-ratio method to...
-
Please help me answer all these question one by one. THE COMPANY IS GOING TO BE WALMART (PLEASE DO NOT USE AND PREVIOUS STUDENT ANSWERS) Remember, one-page memo first. search the web for these...
-
keAssignmentMain.do?invoker=&takeAssignmentSessionLocator=&inprogress=false Print Item Using the data below for the Ace Guitar Company: 6 A Region Sales B Region $500,000 Cost of goods sold $900,000...
-
please answer both parts Winston Company estimates that total factory overhead for the following year will be $979,200. The company has decided that the basis for applying factory overhead should be...

Study smarter with the SolutionInn App


