Question:
The system water(l)/n-pentane(2)/n-heptane(3) exists as a vapor at 101.33 kPa and 100°C with mole fractions z1 = 0.45, z2 = 0.30, z3 = 0.25. The system is slowly cooled at constant pressure until it is completely condensed into a water phase and a hydrocarbon phase. Assuming that the two liquid phases are immiscible, that the vapor phase is an ideal gas, and that the hydrocarbons obey Raoult’s law, determine:
(a) The dewpoint temperature of the mixture and composition of the first condensate.
(b) The temperature at which the second liquid phase appears and its initial composition.
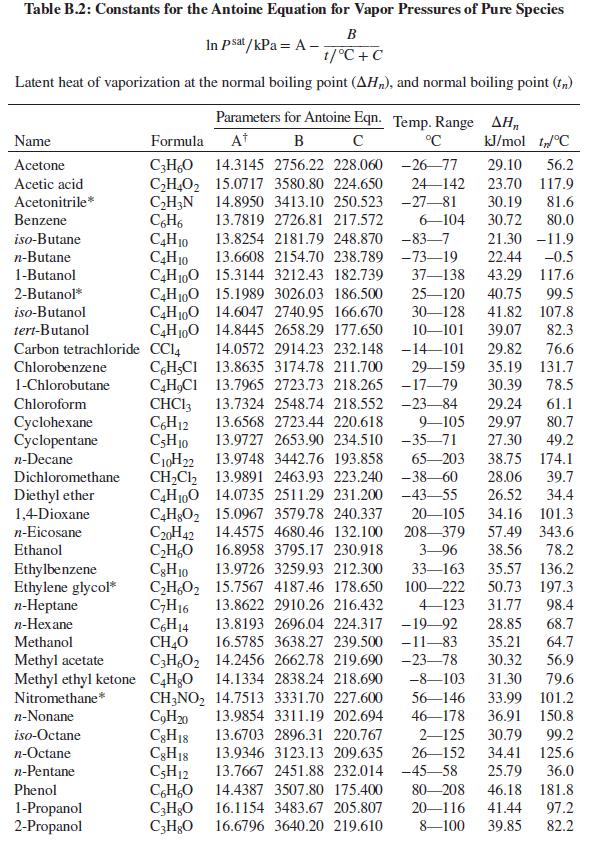
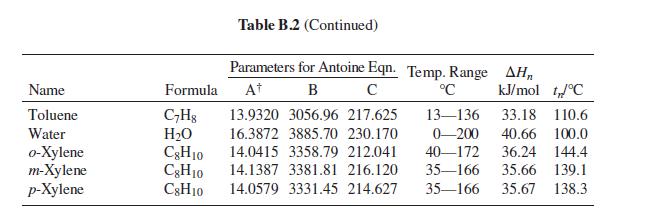
(c) The bubblepoint temperature and the composition of the last bubble of vapor. See Table B.2 for vapor-pressure equations.
Table B.2
Transcribed Image Text:
Table B.2: Constants for the Antoine Equation for Vapor Pressures of Pure Species
B
In Psat /kPa = A -
1/°C +C
Latent heat of vaporization at the normal boiling point (AH„), and normal boiling point (f,)
Parameters for Antoine Eqn. Temp. Range AH,
Name
Formula
At
B
°C
kJ/mol t°C
Acetone
C;H,0 14.3145 2756.22 228.060 -26-77
CH,O2 15.0717 3580.80 224.650
CH;N
CH6
CH10
CH10
C,H100 15.3144 3212.43 182.739
CẠH 100 15.1989 3026.03 186.500
C,H100 14.6047 2740.95 166.670
CH1,0 14.8445 2658.29 177.650
29.10
23.70 117.9
56.2
Acetic acid
24-142
Acetonitrile*
14.8950 3413.10 250.523 -27–81
13.7819 2726.81 217.572
30.19
81.6
Benzene
6–104
30.72
80.0
iso-Butane
13.8254 2181.79 248.870 -83–7
13.6608 2154.70 238.789 -73-19
21.30 -11.9
-0.5
n-Butane
22.44
1-Butanol
37-138
43.29 117.6
2-Butanol*
25–120
40.75
99.5
iso-Butanol
30–128
41.82 107.8
tert-Butanol
10–101
39.07
82.3
Carbon tetrachloride CCI4
Chlorobenzene
1-Chlorobutane
14.0572 2914.23 232.148 -14-101
29.82
76.6
CH5CI 13.8635 3174.78 211.700
C,H,CI 13.7965 2723.73 218.265 -17-79
CHCI3
CH12
CSH10
CioH22 13.9748 3442.76 193.858
CH,Cl, 13.9891 2463.93 223.240 -38-60
C,H100 14.0735 2511.29 231.200 -43-55
C4H3O2 15.0967 3579.78 240.337
C20H42 14.4575 4680.46 132.100 208-379
C,H,0
C3H10
CH,O2 15.7567 4187.46 178.650
C;H16
CH14
CH40
C;H,O2 14.2456 2662.78 219.690 -23-78
29-159
35.19 131.7
78.5
30.39
Chloroform
13.7324 2548.74 218.552 -23-84
29.24
29.97
61.1
80.7
Cyclohexane
Cyclopentane
n-Decane
13.6568 2723.44 220.618
9–105
13.9727 2653.90 234.510 -35--71
27.30
49.2
65-203
38.75 174.1
Dichloromethane
28.06
39.7
Diethyl ether
1,4-Dioxane
26.52
34.4
20–105
34.16 101.3
n-Eicosane
57.49 343.6
Ethanol
16.8958 3795.17 230.918
3–96
38.56
78.2
Ethylbenzene
Ethylene glycol*
n-Heptane
13.9726 3259.93 212.300
33–163
35.57 136.2
100-222
50.73 197.3
98.4
68.7
13.8622 2910.26 216.432
4-123
31.77
п-Нехane
13.8193 2696.04 224.317 -19-92
16.5785 3638.27 239.500 -11-83
28.85
35.21
Methanol
64.7
Methyl acetate
Methyl ethyl ketone C,H3O 14.1334 2838.24 218.690
Nitromethane*
30.32
56.9
-8–103
31.30
79.6
CH;NO, 14.7513 3331.70 227.600
13.9854 3311.19 202.694
56–146
33.99 101.2
36.91
n-Nonane
46–178
150.8
iso-Octane
n-Octane
99.2
C3H18
C3H18
C;H12
13.6703 2896.31 220.767
13.9346 3123.13 209.635
2–125
30.79
26–152
34.41
125.6
n-Pentane
13.7667 2451.88 232.014 -45-58
25.79
36.0
Phenol
14.4387 3507.80 175.400
80–208
46.18 181.8
C;HO
C3H3O
41.44
1-Propanol
2-Propanol
16.1154 3483.67 205.807
20-116
97.2
16.6796 3640.20 219.610
8–100
39.85
82.2







