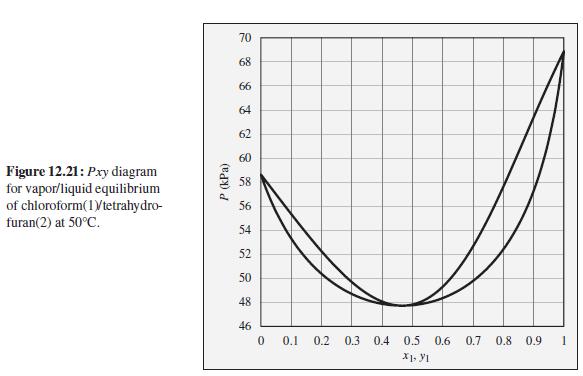
What is the composition of the azeotrope for the chloroform(1)/tetrahydrofuran (2) system? Would this be called a
Question:
What is the composition of the azeotrope for the chloroform(1)/tetrahydrofuran (2) system? Would this be called a high boiling or low-boiling azeotrope?
To the Pxy diagram for chloroform(1)/tetrahydrofuran(2) at 50°C shown in Fig. 12.21.
Transcribed Image Text:
70 68 66 64 62 60 Figure 12.21: Pxy diagram for vapor/liquid equilibrium of chloroform(1Vtetrahydro- furan(2) at 50°C. 58 56 54 52 50 48 46 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 P (kPa)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 86% (15 reviews)
Based on the given Pxy diagram for the chloroform1tetrahydrofuran2 sys...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
What is the composition of the azeotrope for the ethanol(1)/ethyl acetate(2) system? Would this be called a high-boiling or low-boiling azeotrope? Problems 12.3 through 12.8 refer to the Pxy diagram...
-
What is the composition of the FASB's membership?
-
Is the composition of state legislatures in the House of Representatives related to the specific state? Use = 0.05. Perform the following steps. a. State the hypotheses and identify the claim. b....
-
Alpha Mann Ltd makes and sells computer carry bags. Bill Blake, the company accountant, is responsible for preparing the company's annual budget. In compiling the budget data for next year, Blake has...
-
Country A can produce either 10X and 0Y or 0x and 20Y. Country B can produce either 30X and 0Y or 0X and 40Y. Identify the opportunity cost of producing each good for each country. Identify the...
-
The Horizon Company will invest $60,000 in a temporary project that will generate the following cash inflows for the next three years. Year Cash Flow 1 ............ $15,000 2 ............ 25,000 3...
-
List two reasons why, in practice, a project's stand-alone risk is important. AppendixLO1
-
In the current year, Tanager Corporation (a C corporation) had operating income of $480,000 and operating expenses of $390,000. In addition, Tanager had a long-term capital gain of $55,000 and a...
-
1.) If your company is deciding to build a cigarette factory in South Korea, what are the three most important factors to consider in your capital budgeting process? Explain why. 2.) Using the same...
-
Proust Company has FCFF of $ 1.7 billion and FCFE of $ 1.3 billion. Prousts WACC is 11 percent, and its required rate of return for equity is 13 percent. FCFF is expected to grow forever at 7...
-
Consider a closed vessel initially containing 1 mol of pure ethyl acetate at 74C and 100 kPa. Imagine that pure ethanol is slowly added at constant temperature and pressure until the vessel contains...
-
Consider a binary (two-species) system in vapor/liquid equilibrium. Enumerate all of the combinations of intensive variables that could be fixed to fully specify the intensive state of the system.
-
What is the difference between bid shopping and buyout?
-
Problem Statement | Nessie, the wonder-dog, is sprinting at a constant 10.0 m/s in a straight Useful Equations line. After passing point A, she slows to a stop with a constant acceleration of 1.79...
-
1) How much work does a supermarket checkout attendant do on a can of soup he pushes 0.810 m horizontally with a force of 5.60 N? Express your answer in joules and kilocalories. (For each answer,...
-
You are working as a junior developer at Smashing Websites Ltd. You are part of a team of junior developers that have recently joined the organisation. Smashing Websites provide services to a range...
-
3. Consider the system x + y + bz x+by+4z = 2 = 1 ax+ay + 2z == a In each case, determine all values of a and b which give the indicated number of solutions, if possible. If no such a and b exist,...
-
Income statements for Fanning Company for Year 3 and Year 4 follow. FANNING COMPANY Income Statements Year 4. Sales $200,200 Year 3 $180,200 Cost of goods sold. 143,800 121,800 Selling expenses...
-
Oakwood Inc. is a public enterprise whose shares are traded in the over-the-counter market. At December 31, 2018, Oakwood had 6,000,000 authorized shares of $10 par value common stock, of which...
-
For the vector whose polar components are (Vr = 1, Vθ = 0), compute in polars all components of the second covariant derivative Vα;μ;ν. To find...
-
For the acetone (1)/methanol(2) system a vapor mixture for which z1 = 0,25 and z2 = 0.75 is cooled to temperature T in the two-phase region and flow* into a separation chamber at a pressure of 1 bar....
-
For the acetone (1)/methanol(2) system a vapor mixture for which z1 = 0,25 and z2 = 0.75 is cooled to temperature T in the two-phase region and flow* into a separation chamber at a pressure of 1 bar....
-
A process stream contains light species 1 and heavy species 2. A relatively pure liquid stream containing mostly 2 is desired, obtained by a single-stage liquid/vapor separation. Specifications on...
-
Suppose the S&P 500 currently has a level of 960. One contract of S&P 500 index futures has a size of $250 S&P 500 index. You wish to hedge an $800,000-portfolio that has a beta of 1.2. (A)In order...
-
Exhibit 4.1 The balance sheet and income statement shown below are for Koski Inc. Note that the firm has no amortization charges, it does not lease any assets, none of its debt must be retired during...
-
Haley is 57 years of age. She is planning for future long-term care needs. She knows that yearly nursing home costs in her area are currently $69,000, with prices increased by 5 percent annually....

Study smarter with the SolutionInn App


