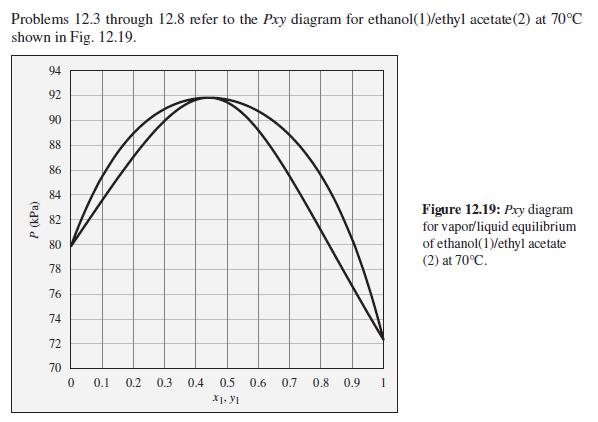
What is the composition of the azeotrope for the ethanol(1)/ethyl acetate(2) system? Would this be called a
Question:
What is the composition of the azeotrope for the ethanol(1)/ethyl acetate(2) system? Would this be called a high-boiling or low-boiling azeotrope?
Transcribed Image Text:
Problems 12.3 through 12.8 refer to the Pxy diagram for ethanol(1)/ethyl acetate(2) at 70°C shown in Fig. 12.19. 94 92 90 88 86 84 Figure 12.19: Pry diagram for vapor/liquid equilibrium of ethanol(1)/ethyl acetate (2) at 70°C. 82 80 78 76 74 72 70 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 X1. y1 P (kPa)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
From the given graph X 1 Y 1 045 X 1 X 2 1 X 2 1X 1 X ...View the full answer

Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
What is the composition of the azeotrope for the chloroform(1)/tetrahydrofuran (2) system? Would this be called a high boiling or low-boiling azeotrope? To the Pxy diagram for...
-
What is the composition of the FASB's membership?
-
Is the composition of state legislatures in the House of Representatives related to the specific state? Use = 0.05. Perform the following steps. a. State the hypotheses and identify the claim. b....
-
Shapiro Inc. was incorporated in 2013 to operate as a computer software service firm with an accounting fiscal year ending August 31. Shapiro's primary product is a sophisticated online...
-
Explain why interest rates differ.
-
Should the auditors prepare adjusting journal entries to correct all errors they discover in the accounting records for the year under audit? Explain.
-
John and Tara Smith are married and have lived in the same home for over 20 years. Johns uncle Tim, who is 64 years old, has lived with the Smiths since March of this year. Tim is searching for...
-
Cooks Department Store advertises that it maintains in its store a barber shop managed by Hunter. Actually, Hunter is not an employee of the store but merely rents space in it. While shaving Jordon...
-
QS 19-8 Predetermined overhead rate LO P3 At the beginning of a year, a company predicts total direct materials costs of $1,080,000 and total overhead costs of $1,350,000. If the company uses direct...
-
We provide you with the balance sheet of a Spanish company at the end of the year. The company carried out its accounting according to the PGC 2007. You have to analyse each of the items and specify...
-
Consider an ethanol(1)/ethyl acetate(2) mixture with x 1 = 0.80, initially at 70C and 80 kPa. Describe the evolution of phases and phase compositions as the pressure is gradually increased to 100...
-
Consider an ethanol(1)/ethyl acetate(2) mixture with x 1 = 0.70, initially at 70C and 100 kPa. Describe the evolution of phases and phase compositions as the pressure is gradually reduced to 70 kPa....
-
In Exercises find all points (if any) of horizontal and vertical tangency to the curve. Use a graphing utility to confirm your results. x = 4 cos , y = 2 sin
-
Below are listed some additional common performance measures not listed in Exhibit 2.1. Which type of employee (senior managers, middle managers, or frontline operations managers) would typically use...
-
If you have a steam distillation system with immiscible organic and water phases plus a vapor phase, two volatile organic compounds plus a nonvolatile organic compound, at equilibrium how many...
-
An auditor is using difference estimation for the confirmation of accounts receivable in the audit of Lafferty Hardware Supply. A random sample of 100 positive confirmations has been sent to...
-
Canterbury Convenience Stores (CCS) is a newly formed organization in Christchurch, New Zealand. It comprises 10 moderately sized convenience stores that previously operated independently of each...
-
Orchard Distributions Pte. Ltd. is a large, Singaporean-based distributor of clothing products to other companies throughout Southeast Asia. Orders are received from customers either by telephone,...
-
Zimmer Company completed its first year of operations on December 31, 2022. Its initial income statement showed that Zimmer had sales revenue of $198,000 and operating expenses of $83,000. Accounts...
-
If the joint cost function for two products is C(x, y) = xy2 + 1 dollars (a) Find the marginal cost (function) with respect to x. (b) Find the marginal cost with respect to y.
-
The energy balance can be developed for just about any process. Since our goal is to learn how to develop model equations as well as to simply apply them, it is valuable practice to obtain the...
-
One mole of an ideal gas (C p =7R/2) in a closed piston/cylinder is expanded from T i = 700 K, P i = 0.75 MPa to P f = 0.1 MPa by the following pathways. For each pathway, calculate U, H, Q, and W EC...
-
The energy balance can be developed for just about any process. Since our goal is to learn how to develop model equations as well as to simply apply them, it is valuable practice to obtain the...
-
Estimate the intrinsic value of the stock company ABC. Dividends were just paid at $8 per share and are expected to grow by 5%. You require 20% on this stock given its volatile characteristics. If...
-
Crane, Inc., a resort management company, is refurbishing one of its hotels at a cost of $6,794,207. Management expects that this will lead to additional cash flows of $1,560,000 for the next six...
-
Match each of the following transactions with the applicable internal control principle that is being violated

Study smarter with the SolutionInn App


