Consider an ethanol(1)/ethyl acetate(2) mixture with x 1 = 0.70, initially at 70C and 100 kPa. Describe
Question:
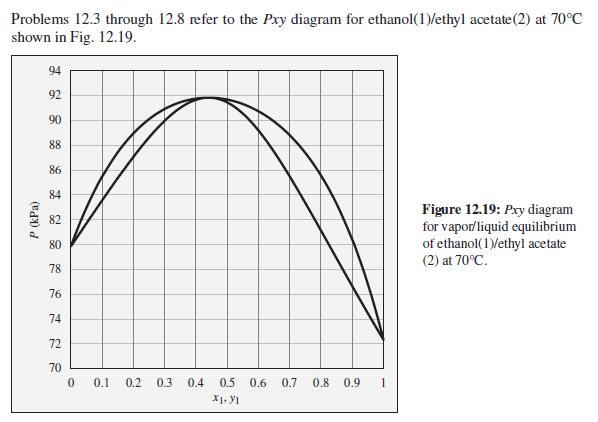
Consider an ethanol(1)/ethyl acetate(2) mixture with x1 = 0.70, initially at 70°C and 100 kPa. Describe the evolution of phases and phase compositions as the pressure is gradually reduced to 70 kPa.
Transcribed Image Text:
Problems 12.3 through 12.8 refer to the Pxy diagram for ethanol(1)/ethyl acetate(2) at 70°C shown in Fig. 12.19. 94 92 90 88 86 84 Figure 12.19: Pry diagram for vapor/liquid equilibrium of ethanol(1)/ethyl acetate (2) at 70°C. 82 80 78 76 74 72 70 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 X1. y1 P (kPa)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
To describe the evolution of phases and phase compositions as the pressure is gradually reduced we need to construct the Pxy diagram for the given eth...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
Describe the evolution of business-to-business (B2B) e-commerce.
-
Describe the evolution of ERP systems.
-
Describe the evolution of American culinary arts.
-
In 2014, Elbert Corporation had net cash provided by operating activities of $531,000; net cash used by investing activities of $963,000; and net cash provided by financing activities of $585,000. At...
-
1. Explain what economists mean when they refer to interest. 2. Listthe components of demand for loanable funds. 3. List the components of supply of loanable funds. 4. Graphically show the loanable...
-
When analytical procedures disclose unexpected changes in financial relationships relative to prior years, the auditors consider the possible reasons for the changes. Give several possible reasons...
-
Rank the following three single taxpayers in order of the magnitude of taxable income (from lowest to highest) and explain your results. Ahmed Baker Chin Gross income $ 90,000 $90,000 $90,000...
-
On June 30, 2007 a flash flood damaged the warehouse and factory of Padway Corporation, completely destroying the work-in-process inventory. There was no damage to either the raw materials or...
-
Tamarisk Corporation amended its pension plan on January 1, 2017, and granted $153,180 of prior service costs to its employees. The employees are expected to provide 2,070 service years in the...
-
Cylinder 1 of diameter 200mm and cylinder 2 of diameter 300 mm are placed in a trough as shown in fig. 3. If cylinder 1 weighs 800 N and cylinder B weight 1200 N, determine the reactions developed at...
-
What is the composition of the azeotrope for the ethanol(1)/ethyl acetate(2) system? Would this be called a high-boiling or low-boiling azeotrope? Problems 12.3 through 12.8 refer to the Pxy diagram...
-
The pressure above a mixture of ethanol and ethyl acetate at 70C is measured to be 78 kPa. What are the possible compositions of the liquid and vapor phases? Problems 12.3 through 12.8 refer to the...
-
A paper released by the World Bank in 2014 states that while economic growth is essential for reducing poverty rates, growth by itself is not enough, and efforts to reduce poverty must be...
-
You are an external auditor in a firm that undertakes the audit of Canadian Life and Mutual (CLM), a large, Montreal-based financial institution. CLM relies heavily on its computer-based information...
-
You need to temporarily increase the feed rate to an existing column without flooding. Since the column is now operating at about \(90 \%\) of flooding, you must vary some operating parameter. The...
-
Consider, again, the clothing data set. Obtain the three summary plots of the sample cross-correlations for lags 1 to 21.
-
Based on the dangling-else discussion in Exercise 3.27, modify the following code to produce the output shown. Use proper indentation techniques. You must not make any additional changes other than...
-
Consider the random process \(U(t)=A\), where \(A\) is a random variable uniformly distributed on \((-1,1)\). (a) Sketch some sample functions of this process. (b) Find the time autocorrelation...
-
Information for two companies in the same industry, Merrill Corporation and Wingate Corporation, is presented here. Instructions Compute free cash flow for both companies and compare. Merrill Wingate...
-
Use this circle graph to answer following Exercises. 1. What fraction of areas maintained by the National Park Service are designated as National Recreation Areas? 2. What fraction of areas...
-
The following liquids, all at atmospheric pressure and 300 K, are mixed: 25 kg of pure water, 40 kg of pure sulfuric acid, and 75 kg of 25-wt-% sulfuric acid. (a) How much heat is liberated if mixing...
-
Ten thousand (10,000) kgh 1 of an 80-wt-% H 2 SO 4 solution in water at 300 K is continuously diluted with chilled water at 280 K to yield a stream containing 50-wt-% H 2 SO 4 at 330 K. (a) What is...
-
What is the composition of the liquid phase in equilibrium with a vapor phase chloroform(1)/tetrahydrofuran(2) mixture of the following compositions at P = 1 bar? (a) y 1 = 0.1 (b) y 1 = 0.2 (c) y 1...
-
Vaughn Company sells two types of pumps. One is large and is for commercial use. The other is smaller and is used in residential swimming pools. The following inventory data is available for the...
-
To fund your dream around-the-world vacation, you plan to save $1,300 per year for the next 14 years starting one year from now. If you can earn an interest rate of 5.83 percent, how much will you...
-
On NSE (Indian stock exchange), shares of ICICI Bank trade for 935 rupees. If the spot exchange rate is USD 0.012, what is the no-arbitrage USD price of ICICI Bank ADR? Assume that transactions costs...

Study smarter with the SolutionInn App


