The pressure above a mixture of ethanol and ethyl acetate at 70C is measured to be 78
Question:
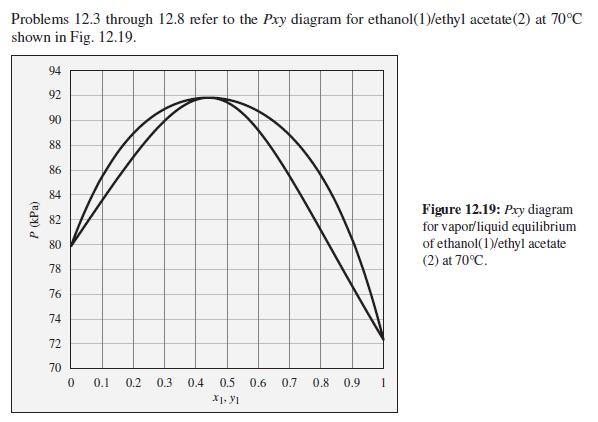
The pressure above a mixture of ethanol and ethyl acetate at 70°C is measured to be 78 kPa. What are the possible compositions of the liquid and vapor phases?
Transcribed Image Text:
Problems 12.3 through 12.8 refer to the Pxy diagram for ethanol(1)/ethyl acetate(2) at 70°C shown in Fig. 12.19. 94 92 90 88 86 84 Figure 12.19: Pry diagram for vapor/liquid equilibrium of ethanol(1)/ethyl acetate (2) at 70°C. 82 80 78 76 74 72 70 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 X1. y1 P (kPa)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
A relativistic rocket is measured to be 50 m long, 2.5 m high, and 2.0 m wide by its pilot. It is traveling at 0.65c (in the direction parallel to its length) relative to an inertial observer. (a)...
-
A mixture of ethanol and 1-propanol behaves ideally at 36C and is in equilibrium with its vapor. If the mole fraction of ethanol in the solution is 0.62, calculate its mole fraction in the vapor...
-
The pressure of an automobile tire is measured to be 190 kPa (gage) before a trip and 215 kPa (gage) after the trip at a location where the atmospheric pressure is 95 kPa. If the temperature of air...
-
Count Dracula, the most famous vampire, rumored to have killed at least 200,000 people, was based on a real person who lived in eastern Europe about 600 years ago. He was indeed a "monster," although...
-
Is it possible for everyones real income to rise even though the income distribution in a society has become more unequal? Prove your answer with a numerical example.
-
Included in the financial statements are a variety of accounting estimates (e.g., allowance for doubtful accounts, obsolete inventory warranty liability). Audit procedures should be designed to...
-
Assume he has no itemized deductions. b. Which engagement maximizes Camerons after-tax cash flow? Explain.
-
In the 1990s, Pfizer, Inc., developed a new antibiotic called Trovan (trovafloxacin mesylate). Tests showed that in animals Trovan had life-threatening side effects, including joint disease, abnormal...
-
Inventory Costing Methods - Feriedic Method The following informarion is for the Bud company for the year: the company sel bjust ore product
-
If $23,000 is invested at 13.5% compounded continuously, what is the amount after 15 years?
-
Consider an ethanol(1)/ethyl acetate(2) mixture with x 1 = 0.70, initially at 70C and 100 kPa. Describe the evolution of phases and phase compositions as the pressure is gradually reduced to 70 kPa....
-
Of the following binary liquid/vapor systems, which can be approximately modeled by Raoults law? For those that cannot, why not? Table B.1 (App. B) may be useful. (a) Benzene/toluene at 1(atm). (b)...
-
Why do free-floating liquids in an orbiting spacecraft adopt a spherical shape?
-
In 2020 the global distribution of sales in the industrial gas industry was as follows: i What is Air Liquides position on a GCI/GRI mapping? Global industrial gas industry 82 billion The 2020 global...
-
The General Social Survey polled a sample of 1048 adults in the year 2010, asking them how many hours per week they spent on the Internet. The sample mean was 9.79 with a standard deviation of 13.41....
-
An article in the Archives of Internal Medicine reported that in a sample of 244 men, 73 had elevated total cholesterol levels (more than 200 milligrams per deciliter). In a sample of 232 women, 44...
-
Explain how search can be used to solve constraint satisfaction problems, such as the eight-queens problem. What difficulties arise when such problems become extremely large (e.g., the...
-
Casse (1981) developed an exercise to encourage his students to develop their empathic skills. He asked them to listen to a recording of a dialogue between John Miller (a US project manager in...
-
Suppose the following is 2022 information for PepsiCo, Inc. and The Coca-Cola Company. Instructions Compute free cash flow for both companies and compare. (S in millions) Net cash provided by...
-
An investor sells a European call on a share for $4. The stock price is $47 and the strike price is $50. Under what circumstances does the investor make a profit? Under what circumstances will the...
-
What is the composition of the vapor phase in equilibrium with a liquid phase chloroform(1)/tetrahydrofuran(2) mixture of the following compositions at P = 1 bar? (a) x 1 = 0.1 (b) x 1 = 0.2 (c) x 1...
-
What is the composition of the liquid phase in equilibrium with a vapor phase ethanol(1)/ethyl acetate(2) mixture of the following compositions at P = 1 bar? (a) y 1 = 0.1 (b) y 1 = 0.2 (c) y 1 = 0.3...
-
What is the composition of the vapor phase in equilibrium with a liquid phase ethanol(1)/ethyl acetate(2) mixture of the following compositions at P = 1 bar? (a) x 1 = 0.1 (b) x 1 = 0.2 (c) x 1 = 0.3...
-
There is a credit rating agency for businesses that gives out various amounts of information based on the subscription level. This company is called a. Business Credit Scoring b. Fair Issue c. Dun...
-
Current Attempt in Progress On July 3 1 , 2 0 2 2 , Crane Compary had a cash balance per books of $ 6 , 2 4 5 . 0 0 . The statement from Dakata State Bark on that date showed a balance of $ 7 , 7 9 5...
-
Cede & Co. expects its EBIT to be $89,000 every year forever. The firm can borrow at 5 percent. Cede currently has no debt, and its cost of equity is 10 percent. If the tax rate is 35 percent, what...

Study smarter with the SolutionInn App


