(a) Describe the processes that transfer carbon from the atmosphere to the land, and from the land...
Question:
(a) Describe the processes that transfer carbon from the atmosphere to the land, and from the land to the atmosphere. What are the chemical reactions that describe these processes?
(b) How do these processes interact to produce the “sawtooth” annual cycle in the atmospheric abundance of CO2 shown in Figure 5.1a?
Figure 5.1a
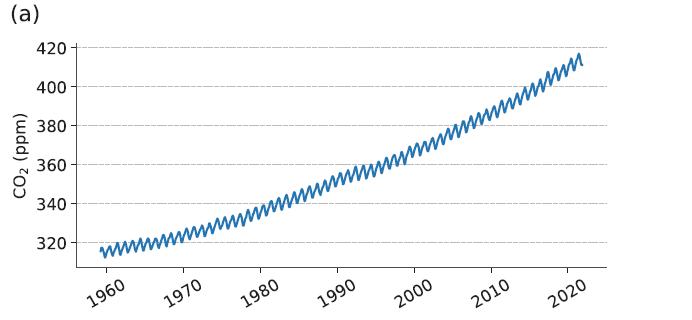
Transcribed Image Text:
(a) CO₂ (ppm) 420 400 380- 360 340 320 www. wwwwwwwwww 1960 1970 1980 1990 2000 2010 m 2020
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a The reaction that transfers carbon from the atmosphere to the land is p...View the full answer

Answered By

Abigael martinez
I have been a tutor for over 3 years and have had the opportunity to work with students of all ages and backgrounds. I have a strong belief that all students have the ability to learn and succeed if given the right tools and support. I am patient and adaptable, and I take the time to get to know each student's individual learning style in order to best support their needs. I am confident in my ability to help students improve their grades and reach their academic goals.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The chapter described how public key authentication is used for message-by-message authentication in digital signatures. However, public key authentication is widely used for initial authentication....
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family. The Incisors own a rental beach house in Hawaii. The beach house was rented for the full year during 2012...
-
When a cosmetic manufacturer tests the market to determine how many women will buy eyeliner that has been tested for safety without subjecting animals to injury, is it involved in a descriptive...
-
In each cycle, an engine removes 150 J from a reservoir at 100 o C and gives off 125 J to a reservoir at 20oC. (a) What is the efficiency of this engine? (b) What is the ratio of its efficiency to...
-
In Fig P2.39 the right leg of the manometer is open to the atmosphere. Find the gage pressure, in Pa, in the air gap in the tank. Neglect surface tension. Air 8 cm 8 cm Oil, SG-0.8 12 cm 9 cm 11 cm...
-
6. Describe the process by which constructive gains on intercompany bonds are realized and recognized on the books of the affiliates. Does recognition of a constructive gain in consolidated financial...
-
Given the aggression scores below for Outcome A of the sleep deprivation experiment, verify that, as suggested earlier, these mean differences shouldnt be taken seriously by testing the null...
-
Selected current year-end financial statements of Cabot Corporation follow. (All sales were on credit, selected balance sheet am at December 31 of the prior year were inventory, $47,900; total...
-
1. Since Mr. Snodgrass doesnt have any historical data, (only an estimate of the number of customers hell serve each month), which specific type of qualitative method is he using? What would be the...
-
A more advanced convection problem: In reality, moist convection ties the atmospheric temperature to the surface temperature. If we assume that the temperature of the atmosphere is always 30K cooler...
-
(a) How much land (in km 2 ) would you need to cover with solar panels in order to generate all of our power from solar energy (assume human power consumption is 15 TW). If one takes into account the...
-
The following table shows the rates of total return in successive years from 2004 to 2008 for the AGF Canadian Stock Fund and for the benchmark Toronto Stock Exchange S&P/TSX Composite Index. By how...
-
What are the major immediate concerns for the HR manager in Austral Group SAA when merging two different organizational cultures - in this case, Peruvian and Norwegian cultures?
-
Explain the relation between the corporate, business and functional strategies. Please produce an in-depth explanation.
-
Consider the problem of terrorism during Radical Reconstruction. If you had been an adviser to the President, how would you propose to deal with the problem? Give a minimum of TWO examples and fully...
-
describe at least one element of an Airport Master Plan. Discuss the importance of this element and how it fits into the overall Airport Master Plan document to include its processes and objectives.
-
It is suggested that Wikipedia has replaced the hardback encyclopedia books, such Encyclopedia Brittanica. What other ways do you foresee technology changing businesses that have been around for...
-
Spherical aluminum powder (SAP) 0.002 mm in diameter is treated to create a thin oxide layer and is then used to produce a SAP dispersion-strengthened material containing 10 vol% Al2O3. Calculate the...
-
Use integration by parts to evaluate the following. Check your answer by taking the derivative. x2e-xdx
-
Single bonds generally experience free rotation at room temperature: Nevertheless, the single bond shown below exhibits a large barrier to rotation. In other words, the energy of the system is...
-
Starting with acetylene as your only source of carbon atoms, identify how you would prepare each member of the following homologous series of aldehydes: a. Ethanal b. Propanal c. Butanal d. Pentanal
-
Starting with acetylene as your only source of carbon atoms, propose a plausible synthesis for 1, 4-dioxane: 1,4-Dioxane
-
Portfolio return and beta Personal Finance Problem Jamie Peters invested $ 1 1 3 , 0 0 0 to set up the following portfolio one year ago: a . Calculate the portfolio beta on the basis of the original...
-
. Emerson Cammack wishes to purchase an annuity contract that will pay him $7,000 a year for the rest of his life. The Philo Life Insurance Company figures that his life expectancy is 20 years, based...
-
Integrity Inc. can sell 20-year, $1,000 par value bonds paying semi-annual interests with a 10% coupon. The bonds can be sold for $1,050 each; flotation cost of $50 per bond will be incurred in this...

Study smarter with the SolutionInn App


