Derive an expression for the fraction of energy received by the surface that comes from the atmosphere
Question:
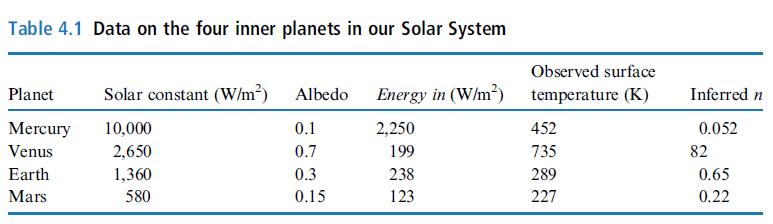
Derive an expression for the fraction of energy received by the surface that comes from the atmosphere (this is the amount of energy that comes from the atmosphere divided by the sum of energy from the Sun and energy from the atmosphere). Using values in Table 4.1, calculate the fraction for Mercury, Earth, and Venus. Make the standard assumption that the atmosphere is transparent to visible photons but opaque to infrared photons.
Table 4.1
Transcribed Image Text:
Table 4.1 Data on the four inner planets in our Solar System Planet Mercury Venus Earth Mars Solar constant (W/m²) Albedo 10,000 2,650 1,360 580 0.1 0.7 0.3 0.15 Energy in (W/m²) 2,250 199 238 123 Observed surface temperature (K) 452 735 289 227 Inferred n 0.052 82 0.65 0.22
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
E in is the amount of energy from the Sun that is falling on the surface The surface temperature ...View the full answer

Answered By

User L
I am FCMA, MBA and LLB with 26 years of experience in Finance, Accounting and Cost Management and Consulting space. Recently I am also cleared all 4 papers of CPA(US) and in the process for complying requirements for license.
I have worked in reputed companies like PwC, Tata Consultancy Services Ltd. I have worked across geographies which includes Germany,UK,Netherlands, Denmark, Ireland, Canada, South Africa, Kazakastan, UAE, Kuwait, Saudi Arabia and India.
All along my corporate career, I ensured that I along with my corporate responsibilities I take coaching and training sessions on subjects I like and enjoy most.
As of now, I have my own consulting firm where Coaching is an important division. I undertake classes for professional courses like CMA,CA, CFA through both classroom and online sessions.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
On Mercury, which has no atmosphere, the difference in temperature between daytime and nighttime temperatures can be 700 K. On the Earth, the difference between daytime and nighttime temperatures can...
-
Assume a planet with a one-layer atmosphere has a solar constant S = 2,000 W/m 2 and an albedo = 0:4. (a) What is the planets surface temperature? Make the standard assumption that the atmosphere is...
-
For a spherical planet with mass M, volume V, and radius R, derive an expression for the acceleration due to gravity at the planets surface, g, in terms of the average density of the planet, r = M/V,...
-
A bank reconciliation takes time and must balance. An employee was struggling in balancing the bank reconciliation. Her supervisor told her to plug (make an unsupported entry for) the difference,...
-
If a 2-kg piece of lead at 100 o C is dropped into a lake at 10 o C, find the entropy change of the universe.
-
The quarter circle gate BC in Fig. P2.86 is hinged at C. Find the horizontal force P required to hold the gate stationary. The width b into the paper is 3 m. water
-
E 7-3 Constructive gain/loss on purchase of subsidiary bonds Abbey Corporation owns 90 percent of the voting common stock of Westminster Corporation. At December 31, 2016, Westminster has $900,000...
-
The manager of a chain of electronics stores is considering running a sale on a Lexmark printer model that contains many popular features. The store buys these printers for $60 apiece and, at the...
-
Why might an analyst prefer to use free cash flow instead of earnings when conducting an absolute valuation? Because free cash flow is usually higher than earnings. Because free cash flow represents...
-
Cherry Cotta makes custom ordered clay pots for residential gardens. Below is cost information regarding its latest job. a. Materials were purchased on account. $18,996 purchased b. A materials...
-
As we will discover in Chapter 11, one way to solve global warming is to increase the reflectivity of the planet (I will explain how in Chapter 11). To reduce the Earths temperature by 1 K, how much...
-
Assume a planet with a one-layer atmosphere and values of solar constant S = 1,000 W/m 2 and albedo = 0:25. Let us assume there is some dust in the atmosphere, so that 50 percent of the Suns energy...
-
The highest and lowest birth rates in the United States in 2008 were in Utah and Maine, respectively. Utah reported 55,633 births with a population of 2,736,424 people. Maine reported 13,610 births...
-
The Buckle, Inc., operates 387 stores in 39 states, selling brand name apparel like Lucky jeans and Fossil belts and watches. Some of the items included in its 2008 statement of cash flows presented...
-
Assume that on July 1, 2011, Big Corp. loaned Little Corp. \(\$ 12,000\) for a period of one year at 6 percent interest. What amount of interest revenue will Big report for 2011? What amount of cash...
-
A vacuum column with 25 real stages is operating with a pressure drop of \(0.3 \mathrm{in}\). of water per stage. Assume pressure drop in the condenser and the reboiler is 0.6 in. of water each. The...
-
You want to determine the viscosity of an oil which has an SG of 0.9. To do this, you drop a spherical glass bead $(\mathrm{SG}=2.7)$ with a diameter of $0.5 \mathrm{~mm}$ into a large vertical...
-
Show that 673 - 356 can be computed by adding 673 to the 10's complement of 356 and discarding the end carry. Draw the block diagram of a three-stage decimal arithmetic unit and show how this...
-
We prepare several epoxy-matrix composites using different lengths of 3-m- diameter ZrO2 fibers and find that the strength of the composite increases with increasing fiber length up to 5 mm. For...
-
What is the maximum volume of 0.25 M sodium hypochlorite solution (NaOCl, laundry bleach) that can be prepared by dilution of 1.00 L of 0.80 M NaOCl?
-
For each of the following IR spectra, determine whether it is consistent with the structure of a ketone, an alcohol, a carboxylic acid, a primary amine, or a secondary amine. a. b. c. d. e. f. 100-...
-
Carefully consider the structure of 2, 3-dimethyl-2-butene. There are twelve C sp3 H bonds, and they are all identical. Nevertheless, there is more than one signal just to the right of 3000 cm -1 in...
-
Match each compound with the appropriate IR spectrum: a. b. c. d. e. f. `NH2 100- 60- 20- 4000 3500 3000 2500 2000 1500 1000 Wavenumber (cm-1) % Transmittance
-
question 6 Timely Inc. produces luxury bags. The budgeted sales and production for the next three months are as follows july. august september Sales, in units 1,115. 1229. 1302 Production. in units...
-
On May 12 Zimmer Corporation placed in service equipment (seven-year property) with a basis of $220,000. This was Zimmer's only asset acquired during the year. Calculate the maximum depreciation...
-
Power Manufacturing has equipment that it purchased 7 years ago for $2,550,000. The equipment was used for a project that was intended to last for 9 years and was being depreciated over the life of...

Study smarter with the SolutionInn App


