Question: In molecular and solid-state applications, one often uses a basis of orbitals aligned with the cartesian axes rather than the basis used throughout this chapter.
In molecular and solid-state applications, one often uses a basis of orbitals aligned with the cartesian axes rather than the basis used throughout this chapter. For example, the orbitals
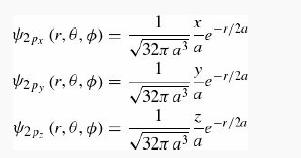
are a basis for the hydrogen states with n = 2 and ℓ = 1.
(a) Show that each of these orbitals can be written as a linear combination of the orbitals Ψnℓm with n = 2, ℓ = 1and m = 1,0,1.
(b) Show that the states Ψ2pi are eigenstates of the corresponding component of angular momentum: L̂i. What is the eigenvalue in each case.
(c) Make contour plots (as in Figure 4.9) for the three orbitals. In Mathematica use ContourPlot3D.
Figure 4.9
2. (r,, ) = 2p, (r,, ) = 2p. (r,6,) = 1 e V32 1 V32 1 X 323 -1/2a -/2a
Step by Step Solution
3.47 Rating (163 Votes )
There are 3 Steps involved in it
a A similar calculation gives b L z 2pz L z 210 0 since m 0 By cyclic perm... View full answer

Get step-by-step solutions from verified subject matter experts


