A vessel contains a liquid and a vapor phase in equilibrium at a pressure of 0.1 atm.
Question:
A vessel contains a liquid and a vapor phase in equilibrium at a pressure of 0.1 atm. The vapor phase contains 100 moles of species A and 200 moles of species B. The liquid phase contains 500 total moles of species. The saturation pressure of species A is 0.1 atm and of B is 0.5 atm. Both the vapor and the liquid phases behave ideally!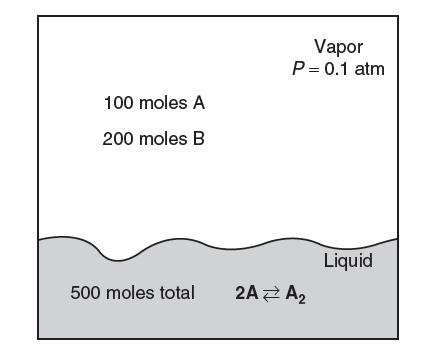
Species A dimerizes in the liquid phase and A2 is completely involatile.
(a) Calculate the equilibrium constant for the dimerization reaction.
(b) Calculate the values for the number of moles of A, B, and A2 in the liquid phase.
(c) A colleague maintains that the dimerization reaction does not occur in the liquid phase; rather, he believes that the liquid phase behaves nonideally. If species A occurs only as a monomer (as A, not A2) as he thinks, how many moles of species A would exist in the liquid phase [keep the total number of A atoms in the liquid phase the same as for part (b)]?
(d) Given your colleague’s model in part (c), what value of γB is necessary for his model to fi t the data?
(e) Can you explain nonideality in phase equilibria solely by introducing an association reaction to an ideal vapor–liquid system? Can an association reaction be used to explain negative (γB > 1) deviations from ideality?
Step by Step Answer:






