Gaseous hydrogen can be produced by the steam cracking of methane in a catalytic reactor at 500C
Question:
Gaseous hydrogen can be produced by the steam cracking of methane in a catalytic reactor at 500°C and 1 bar according to the following reaction: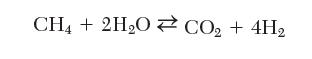
(a) If 5 moles of steam are fed into the reactor for every mole of methane, at equilibrium, how many moles of hydrogen are produced?
(b) If we build a reactor and run it under these conditions, could we ever get a lower conversion (less hydrogen) than that calculated in part (a)? Can we ever get a higher conversion? Explain.
(c) Would it make sense to increase the pressure in order to increase the equilibrium conversion?
Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





