At 1100 K, solid SiO2 has values of enthalpy and entropy of 2856.84 3kJ/mol4 and 124.51 [J/(mol
Question:
At 1100 K, solid SiO2 has values of enthalpy and entropy of 2856.84 3kJ/mol4 and 124.51 [J/(mol K)], respectively. At 2500 K, liquid SiO2 has values of enthalpy and entropy of 2738.44 [kJ/mol] and 191.94 [J/(mol K)], respectively. The heat capacities for the solid and liquid phases are given by:![]()
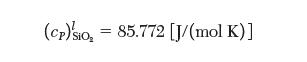
Using only these data, determine the temperature of the phase transition between solid and liquid.
What is the enthalpy of fusion?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





