Solid sulfur undergoes a phase transition between the monoclinic (m) and orthorhombic (o) phases at a temperature
Question:
Solid sulfur undergoes a phase transition between the monoclinic (m) and orthorhombic (o) phases at a temperature of 368.3 K and pressure of 1 bar. Calculate the difference in Gibbs energy between monoclinic sulfur and orthorhombic sulfur at 298 K and a pressure of 1 bar. Which phase is more stable at 298 K? Take the entropy in each phase to be given by the following expressions: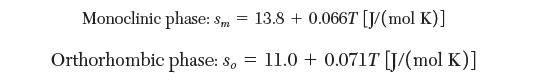
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





