Below is a plot of the natural log of the fugacity coeffi cient, ln (i) , of
Question:
Below is a plot of the natural log of the fugacity coeffi cient, ln (φi) , of pure NH3 as a function of pressure at a temperature of 100°C. From this plot, as best you can, determine the molar volume of this species at 500 bar and 100°C.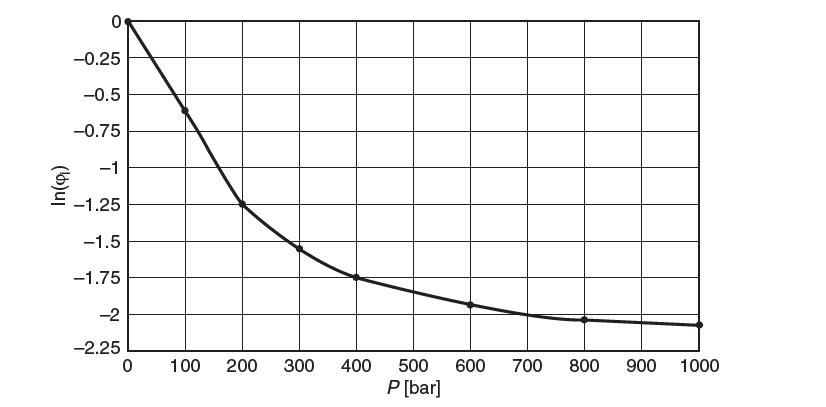
Transcribed Image Text:
(b)ul -0.25 -0.5 -0.75 -1 -1.25 -1.5 -1.75 -2 -2.25 0 100 200 300 400 500 P [bar] 600 700 800 900 1000
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
Below is a plot of the natural log of the activity coeffi cients ( ln i) a binary liquid mixture of species a and b vs. mole fraction of species a (xa) at 300 K. (a) What is the reference state for...
-
Question 3. Find the second derivative of y = x - 1 Question 4. If f(x) = x-1 Find f'(-2).
-
Jason and Mary Wells, friends of yours, were married on December 30, 2020. They know you are studying taxes and have sent you an e-mail with a question concerning their filing status. Jason and Mary...
-
Imagine that you are a financial manager researching investments for your client. Use the Strayer Learning Resource Center to research the stock of any U.S. publicly traded company that you may...
-
Suppose the trade-off theory of capital structure is true. Can you predict how companies debt ratios should change over time? How do these predictions differ from the pecking-order theorys?
-
How would you describe the findings of at least one important piece of research on achieving high-performance in public organizations? What are its implications for the practice of public management?...
-
Consider the following data for the assembly division of Cranberry Watches, Inc., The assembly division uses the weighted- average method of process costing. aDegree of completion: direct materials,...
-
15 Edge Company produces two models of its product with the same machine. The machine has a capacity of 172 hours per month. The following information is available. Standard $ 230 100 $ 130 Selling...
-
A gas mixture containing an equal number of moles of species 1 and 2 at 300 K and 30 bar perfectly obeys the Lewis fugacity rule. Calculate the Gibbs energy of mixing.
-
The following data are available for ethylene at 24.95C. From these data, estimate the fugacity and the fugacity coeffi cient of ethylene at 50.5 bar and 24.95C. P [bar] 1.0 5.1 10.1 15.2 20.2 25.3...
-
Find h' in terms of f' and g'. h(x) = f(g(sin 4x))
-
The cable supports two cylinders as shown. Cylinders E and F have a mass of 15 kg and 35 kg, respectively. Determine the sag dc and the tension in each segment of the cable. 2 m 2.5 m -2.5m- 2 m dc E...
-
A raft foundation having dimensions of 35 m x 35 m in plan is to be constructed on a deep deposit of sand. Foundation depth and the ground water table are both 5 m below the surface. Unit weight of...
-
Determine the number of 2 X 4 @ 92 5/8" studs needed for the garage in Figures 14.63 and 14.64. The studs are spaced 16 inches on center. Add two studs for each door and corner. Ignore the gable ends...
-
Sketch a cumulative flow diagram that represents the growth and dissipation of a rush hour period at a toll bridge with time-independent capacity. 1) Identify on the diagram: the arrival curve A(t),...
-
Plot the reciprocal lattice for a polycrystalline sample o fa material with a simple tetragonal structure and lattice parameters a = 4.0 A and c = 5.0 A. (Use a two dimensional section through the...
-
Describe an example of an interactive pricing activity that would be appropriate when price is used as a background variable. Then describe an example of an interactive pricing activity that would be...
-
Arlington Merchants reported the following on its income statement for the fiscal years ending December 31, 2016 and 2015. 2016 2015 Sales $4,857,500 $4,752,900 Cost of goods sold 3,258,950 3,207,000...
-
In anaerobic bacteria, the source of carbon may be a molecule other than glucose and the final electron acceptor is some molecule other than 02 Could a bacterium evolve to use the ethanol nitrate...
-
The standard potentials of proteins are not commonly measured by the methods described in this chapter because proteins often lose their native structure and function when they react on the surfaces...
-
Nitric acid hydrates have received much attention as possible catalysts for heterogeneous reactions that bring about the Antarctic ozone hole. Worsnop et al. investigated the thermodynamic stability...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...

Study smarter with the SolutionInn App


