Calculate the van der Waals parameter a for CH4, C6H6, and CH3OH. Based on these values, estimate
Question:
Calculate the van der Waals parameter a for CH4, C6H6, and CH3OH. Based on these values, estimate the value of C6 for each species. Compare the values obtained with that calculated by Equation (4.8).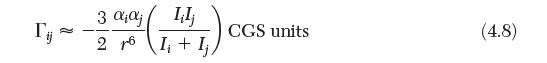
Transcribed Image Text:
3 ;j Lilj 26 I+Ij CGS units a (4.8)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Calculate the van der Waals parameter b for CH4, C6H6, and CH3OH. Based on these values, estimate the molecular diameter of each species. Compare the values obtained with those in Table 4.2. TABLE...
-
Van der Waals Equation and Critical Points (a) In p V- diagrams the slope p/V along an isotherm is never positive. Explain why. (b) Regions where p/V = 0 represent equilibrium between two phases;...
-
Calculate the van der Waals parameters of carbon dioxide from the values of the critical constants and compare your results with the values for and b in Table 7.4. Table 7.4 RedlichKwong van der...
-
Let F = [F] lb. a) Determine the force Fp acting at roller D. b) Determine the force FE acting at pin E. F 4 ft B 600 lb. ft -3 ft C -3 ft OD -2 ft- E
-
Analyze the debit and credit aspect of each transaction listed at (a), (b) and (c) of question B3.2? (a) Owner puts cash into the business. (b) Buy a vehicle for cash. (c) Receive a bill for...
-
The personnel department of a certain industrial firm used 12 subjects in a study to determine the relationship between job performance rating (y) and scores of four tests. The data are as follows:...
-
CI4.5. Whenis the forecasted growthrate in residual operating income the sameas the forecasted growth ratein sales?
-
Pike Seafood Company purchases lobsters and processes them into tails and flakes. It sells the lobster tails for $20 per pound and the flakes for $15 per pound. On average, 100 pounds of lobster are...
-
Job A3B was ordered by a customer on September 25. During the month of September, Jaycee Corporation used $1,700 of direct materials and used $3,200 of direct labor. The job was not finished in...
-
Consider a cylinder fi tted with a piston that contains 2 mol of H2O in a container at 1000 K. Calculate how much work is required to isothermally and reversibly compress this gas from 10 L to 1 L,...
-
Using data from Table 4.2, estimate the equilibrium bond length that would exist in a molecule of Xe2. TABLE 4.2 Lennard-Jones Parameters for Several Species /k(K) 10.2 35.7 Gas He H CH4 C6H6 288 28...
-
Rose, Inc., of Dallas, Texas, needed to infuse capital into its foreign subsidiaries to support their expansion. As of August 2001, it planned to issue stock in the United States. However, after the...
-
PART 1: DIGITAL TECHNOLOGY: Describe the key digital technology groups studied in this course and include a discussion of two examples for each group. PART 2: SOCIAL MEDIA: As studied in this course,...
-
Doing a strategic analysis of GraceKennedy Limited, What is the current level of its economic performance, an indication of the factors responsible for the current performance and recommendations for...
-
Dynamic capability is the ability for change and manage corporate learning. It allows an enterprise to adapt, develop and respond to future opportunities and discontinuous technologies. Innovation...
-
What potential solutions can organizations try to help support the adoption of a CDSS? In other words, what are some ways an organization can address the factors of implementation obstruction that...
-
Identify and briefly describe and discuss the three most important factors in building and maintaining trust among virtual global team members. Include in your discussion how you can leverage these...
-
If planned expenditures are below actual production, what will happen to income? Explain the process by which this happens.
-
What is your assessment of the negotiations process, given what you have studied? What are your recommendations for Mr. Reed? You must justify your conclusions
-
Outline a reasonable mechanism for the formation of tert-butyl methyl ether according to the preceding equation.
-
Write equations describing two different ways in which benzyl ethyl ether could be prepared by a Williamson ether synthesis.
-
Only one combination of alkyl halide and alkoxide is appropriate for the preparation of each of the following ethers by the Williamson ether synthesis. What is the correct combination in each case?
-
How to solve general ledger cash balance chapter 9 assignment 5
-
On 31 July 2018, Sipho bought 1 000 ordinary shares in ABC Ltd at a cost of R2 750. On 31 December 2018 the company made a 1 for 10 bonus issue. On 31 March 2019, Sipho sold 300 shares for R800. What...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%

Study smarter with the SolutionInn App


