Consider a binary mixture of n-propanol and water in vaporliquid equilibrium (VLE). Let n-propanol be designated species
Question:
Consider a binary mixture of n-propanol and water in vapor–liquid equilibrium (VLE). Let n-propanol be designated species 1 and water, species 2. A plot of the activity coeffi cients for this system at 100°C follows. The Lewis/Randall reference state is chosen for both species. The mole fraction of n-propanol in the liquid, x1, is 0.2, and the temperature is 100°C. The saturation pressure of n-propanol at 100°C is 1.12 bar.
(a) Label the curve that corresponds to the activity coeffi cient for n-propanol, γ1, and the curve that corresponds to the activity coeffi cient for water, γ2. Explain.
(b) Are like or unlike interactions stronger? Explain.
(c) Find the total pressure of the system.
(d) Find the mole fraction of n-propanol in the vapor phase.
(e) Estimate the value of the Henry’s law constant of n-propanol in water, H1.
(f) Does this system exhibit an azeotrope? Explain.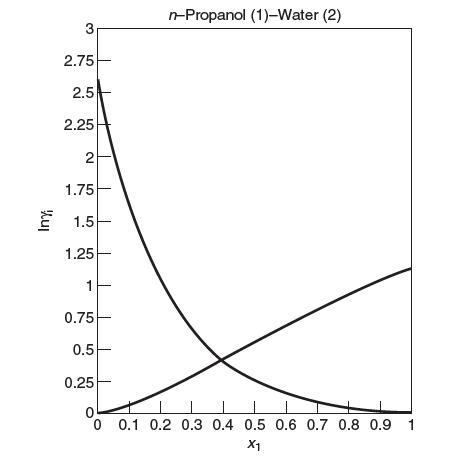
Step by Step Answer:





