Consider the equilibrium between copper and its oxide: The Gibbs energy of formation of Cu2O is given
Question:
Consider the equilibrium between copper and its oxide: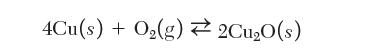
The Gibbs energy of formation of Cu2O is given by![]()
Make a plot of pO2 vs. T, illustrating where Cu is stable and where Cu2O is stable in the temperature range of 300 K to 1300 K.
Transcribed Image Text:
4Cu(s) + O(g) 2Cu0(s)
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
The equilibrium pressure of H, over solid uranium and uranium hydride, UH3' at 500 K is 139 Pa. Calculate the standard Gibbs energy of formation ofUH3 (s) at 500 K.
-
The Soviet Venera VII probe, which reached Venus on December 15, 1970, found the following conditions on the planet surface: Interferometric measurements indicate that there is only a 10 to 20 K...
-
The following data represents the number of Grand Jury indictments for Gloucester County, New Jersey for a sample of 11 weeks selected from July 2010 through June 2011 as reproduced from that...
-
Suppose that the Medicare rate of hospital reimbursement is reduced. Explain why the costs may not be shifted to other patients in the short run.
-
How might the concept of Crowd funding impact the traditional donor relationship development process? What implications might this have on the organization's fundraising ability in the long-run?
-
A rope with length L and mass density per unit length is suspended vertically from one end. Find the tension as a function of height along the rope.
-
Calculate the coefficient of determination for the drug reaction example. The data are repeated in Table 11.4 for convenience. Interpret the result. LO9
-
A statement of financial affairs created for an insolvent corporation that is beginning the process of liquidation discloses the following data (assets are shown at net realizable values): Assets...
-
Question 2: Following are the selected transactions related to the business of Aslam Stores during January 2002. 1. Purchased merchandise for Cash and on credit from Mr. Ali for Rs. 40000 and Rs....
-
Consider the reaction of CrCl2 with H2 to form solid Cr as follows: At Answer the following questions: (a) From these data, estimate the enthalpy of reaction. (b) In an attempt to increase the extent...
-
Hydrogen cyanide can be manufactured by reaction of acetylene and nitrogen: Calculate the equilibrium composition at 800 K and 1 bar. CH + N 2HCN
-
Give a practical argument against letting government borrow to finance any capital expenditure.
-
Ranjha Inc. manufactures widgets. The end product is produced in different departments within the plant. One component, C1, is causing some concern. The component is integral to the production of...
-
. Write a Java program in NetBeans that creates a LinkedHashSet. Your Java program must use the methods in the LinkedHashSet interface to do the following: 2.1 Add the above elements into the...
-
on the following statement: Mona is an industrial engineer working for car parts manufacturing facility. She collected the following data on three alternatives of sustainable energy systems to be...
-
Alvarado Company produced 2,900 units of product that required 6 standard direct labor hours per unit. The standard fixed overhead cost per unit is $2.55 per direct labor hour at 16,200 hours, which...
-
Find the complexity of the function given below. void function(int n) { int i, count =0; for(i=1; i*i
-
What is the difference between a positive differentiating factor and a negative one? Is it possible for an item to simultaneously have both positive and negative differentiating factors?
-
The following data are supplied for the common stocks of Nikola Corporation, Tesla, Inc. and General Motors: Nikola Corp (NKLA) Tesla Inc. (TSLA) Close Price ($) Close Price ($) 67.53 30.00 40.81...
-
The total cross-sections for reactions between alkali metal atoms and halogen molecules are given in the table below (R.D. Levine and R.B. Bernstein, Molecular reaction dynamics, Clarendon Press,...
-
For the thermal decomposition Of F2O by the reaction 2 F2O (g) 2 Fl (g) + 0z (g),) Czarnowski and H) Schumacher (Chem. Phys. Lett. 17, 235 (1972)) have suggested the following mechanism: (a) Using...
-
One of the most historically significant studies of chemical reaction rates was that by M. Bodenstein (z. physik. Chem. 29,295 (1899)) of the gas-phase reaction 2 Hl(g) -t H2(g) + I2(g) and its...
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...
Complete Financial Planner Budget And Financial Organizer 1st Edition - ISBN: 1707919003 - Free Book

Study smarter with the SolutionInn App


