Consider the well-insulated container shown below. Two gases, gas A and gas B, are separated by a
Question:
Consider the well-insulated container shown below. Two gases, gas A and gas B, are separated by a metallic piston. The piston is initially held in place by a latch 10 cm from the left of the container.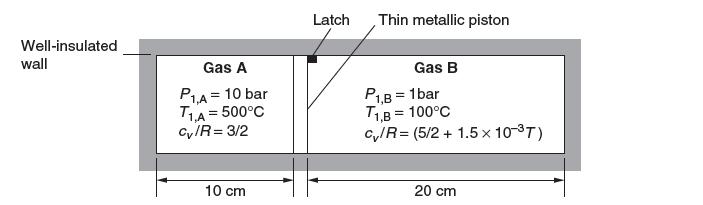
Gas A, which is located in the left compartment, is initially at 10 bar and 500°C. The heat capacity of gas A is constant: (cv,A/R) = 3/2. Gas B is located in the right compartment and is initially at 1 bar and 100°C. The heat capacity of gas B is given by (cv,B/R) = 5/2 - 1.5 * 10-3 T where T is in Kelvin. You may use the ideal gas model for both gases.
(a) The latch is removed and the piston moves until the pressure and temperature in the two compartments become equal. What are the fi nal pressure and temperature? State any assumptions that you make.
(b) Calculate the entropy change of the universe. Is this process possible?
Step by Step Answer:






