Determine the second and third virial coeffi cients using RedlichKwong equation of state. 1 1- x =
Question:
Determine the second and third virial coeffi cients using Redlich–Kwong equation of state.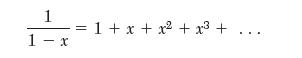
Transcribed Image Text:
1 1- x = 1 + x +x + 3 +
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Rayan Gilbert
I have been teaching since I started my graduation 3 years ago. As a student, working as Teacher/PA has been tough but made me learn the needs for student and how to help them resolve their problems efficiently. I feel good to be able to help out students because I'm passionate about teaching. My motto for teaching is to convey the knowledge I have to students in a way that makes them understand it without breaking a sweat.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Determine the second and third virial coeffi cients using the van der Waals equation of state. Begin by writing the van der Waals equation in compressibility factor form and performing a power-series...
-
Using the link above, could you please summarize the case. And determine who is witness, victim, suspect, accused. https://www.canlii.org/en/on/onsc/doc/2013/2013onsc7865/2013onsc7865.html Using the...
-
Polymer scientists often report their data in rather strange units. For example, in the determination of molar masses of polymers in solution by osmometry, osmotic pressures are often reported in...
-
Three charges are placed as shown in the figure below. A scale is provided for the distances. Match the numerical values with the appropriate force of interaction. 19=8c a. b. 9=-2C I'm 93=4...
-
Explain what is meant by each of the following: (a) Comparability; (b) Understandability; and (c) Materiality?
-
An experiment was designed for the Department of Materials Engineering at Virginia Polytechnic Institute and State University to study hydrogen embrittlement properties based on electrolytic hydrogen...
-
C14.2. Avaluation thatsimply capitalizes a forecast of operating income forthenextyear implicitly assumes that residual operating income willcontinue as a perpetuity. Is this correct?
-
Break-Even Assume a firm is considering a new project that requires an initial investment and has equal sales and costs over its life. Will the project reach the accounting, cash, or financial...
-
Please I need the Journal entries, Adjusted Journal entries, T-Accounts (assets=liabilities+owner equity), trial balance and Financial (income statement, Retained Earnings Statement and the balance...
-
The Dieterici equation of state is given by: (a) Find an expression for the parameters a and b in terms of the critical properties Tc and Pc. (b) Find the compressibility factor at the critical...
-
Consider a cylinder fi tted with a piston that contains 2 mol of H2O in a container at 1000 K. Calculate how much work is required to isothermally and reversibly compress this gas from 10 L to 1 L,...
-
Commodity prices have been decimated in 2015: natural gas down 38.5% and nickel down nearly 43%. The United States imports nickel from Canada and exports natural gas to Mexico. How do these price...
-
How do emergent properties within teams, such as synergy and collective intelligence, manifest and influence team performance, and what factors contribute to their development and sustenance?
-
Think about your workplace, organization, or industry; if you are not currently working, think about previous employment or a job that you are aiming for. The broader your perspective, the more...
-
Choose any global organization that successfully undertook a strategic transformation to adapt to changing market dynamics and sustain its competitive advantage. Examine the company's challenges,...
-
In examining C&C Sports through the lens of a SWOT analysis, several key factors come to light. The strengths of the company are evident in its established brand reputation and a loyal customer base...
-
A perfectly insulated container initially contains 0.2 kg of ice at -15 C. Now we add water at 30 C, but only the minimum amount needed to barely melt all the ice. Find the net entropy change of the...
-
What is the current state of U.S. fiscal policy? Would you advise the United States to change its fiscal policy? Why?
-
Jax Incorporated reports the following data for its only product. The company had no beginning finished goods inventory and it uses absorption costing. $ 57.30 per unit $ 10.30 per unit $ 7.80 per...
-
The following conversion has been reported in the chemical literature. It was carried out in two steps, the first of which involved formation of a p-toluenesulfonate ester. Indicate the reagents for...
-
Sometimes the strongly basic properties of Grignard reagents can be turned to synthetic advantage. A chemist needed samples of butane specifically labeled with deuterium, the mass 2 isotope of...
-
Diphenylmethane is significantly more acidic than benzene, and triphenylmethane is more acidic than either. Identify the most acidic proton in each compound, and suggest a reason for the trend in...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


