Determine the second and third virial coeffi cients using the van der Waals equation of state. Begin
Question:
Determine the second and third virial coeffi cients using the van der Waals equation of state.
Begin by writing the van der Waals equation in compressibility factor form and performing a power-series expansion. The following mathematical relation is useful: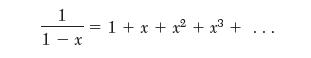
Transcribed Image Text:
1 1- x = 1+ x +x + x +
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
In this problem we seek to develop an expression for the van der Waals constants a and b in terms of molecular parameters using the Sutherland model for potential energy. (a) Show that writing the...
-
Now assume that there are 1,500 identical firms in this competitive industry. That is, there are 1,500 firms, each of which has the cost data shown in the table. Complete the industry supply schedule...
-
The van der Waals equation of state, an approximate representation of the behavior of gases at high pressure, is given by Where a and b are constants having different values for different gases. (In...
-
Find the net torque on the wheel in the figure below about the axle through O perpendicular to the page, taking a = 7.00 cm and b = 25.0 cm. (Indicate the direction with the sign of your answer....
-
Explain what is meant by each of the following: (a) Freedom from error (b) Neutrality; (c) Completeness;
-
A set of experimental runs was made to determine a way of predicting cooking time y at various levels of oven width x 1 , and flue temperature x 2 . The coded data were recorded as follows. Estimate...
-
C14.3. What is thedifference between anSF2andan SF3 forecast?
-
Farrah and Davidson had beginning capital balances of $25,000 and $20,000, respectively. The two partners fail to agree on a profit-and-loss ratio. For the first month (June 2012), the partnership...
-
You have accumulated some money for your retirement. You are going to withdraw $71,154 every year at the end of the year for the next 19 years. How much money have you accumulated for your...
-
Determine the second and third virial coeffi cients using RedlichKwong equation of state. 1 1- x = 1 + x +x + 3 +
-
Consider a cylinder fi tted with a piston that contains 2 mol of H2O in a container at 1000 K. Calculate how much work is required to isothermally and reversibly compress this gas from 10 L to 1 L,...
-
c = 0.80, x = 20.6, = 4.7, n = 100 Construct the indicated confidence interval for the population mean . If convenient, use technology to construct the confidence interval.
-
Analysts and investors often use return on equity ( ROE ) to compare profitability of a company with other firms in the industry. ROE is considered a very important measure, and managers strive to...
-
Provide a brief summary of the case. Respond to the following: 1. Discuss the factors which contributed to the success of the change process in terms of unfreeze, move, and refreeze stages in force...
-
Prepare a proposal where a government agency meets with consumer groups and producers on how to address the shortages in rice, sugar, onions, and fuel, i.e. oil, gasoline and the like. Use the format...
-
Decided to embark on a personal improvement project centered around time management after reviewing the insightful workbook by Neuhauser et al. (2004). My decision was influenced by my recognition...
-
You are the Senior Manager of IAuditYou LLP, you were recently assigned to take over a very important client for the company, The engagement partner, Max Roff, has been the audit partner for the past...
-
The marginal propensity to expend is .66 and autonomous expenditures have just fallen by $20. a. What will likely happen to equilibrium income? b. Demonstrate graphically.
-
2.) Find the Laplace transform of f(t) 7e-St cos 2t +9 sinh2 2t. Use Laplace Table. %3D
-
The 18-electron rule is a general, but not universal, guide for assessing whether a certain transition-metal complex is stable or not. Both of the following are stable compounds, but only one obeys...
-
One of the main uses of the linear -olefins prepared by oligomerization of ethylene is in the preparation of linear low-density polyethylene. Linear low-density polyethylene is a copolymer produced...
-
Each of the following ethers has been shown to be or is suspected to be a mutagen, which means it can induce mutations in test cells. Write the structure of each of these ethers. (a) Chloromethyl...
-
help me A 35% discount on 3 smart phone amounts to $385. What is the phone's list price? Answer =$ (rounded to the nearest cent)
-
What effect is there on the income statement and balance sheet when an expense is left too long as a liability
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid

Study smarter with the SolutionInn App


