If the diatomic gas of Problem 3.47 were nonideal at the pressures in the problem and attractive
Question:
If the diatomic gas of Problem 3.47 were nonideal at the pressures in the problem and attractive forces dominate, qualitatively describe how the fi nal temperature in tank A would change from the answer you obtained in that problem.
Problem 3.47
Consider the system shown below. Tank A has a volume of 0.3 m3 and initially contains an ideal diatomic gas at 700 kPa, 40°C. Cylinder B has a piston resting on the bottom, at which point the spring exerts no force on the piston. The piston–cylinder has a cross-sectional area of 0.065 m2, the piston has a mass of 40 kg, and the spring constant is 3500 N/m. Atmospheric pressure is 100 kPa.
Tanks A and B are well insulated and do not transfer heat between each other. The valve is opened and gas fl ows into the cylinder until pressures in A and B become equal and the valve is closed.
You may assume constant heat capacity. Determine the fi nal pressure in the system. Assuming the gas in A has undergone a reversible, adiabatic expansion, fi nd the fi nal temperature in cylinder A. The temperatures in tank A and B are not necessarily equal.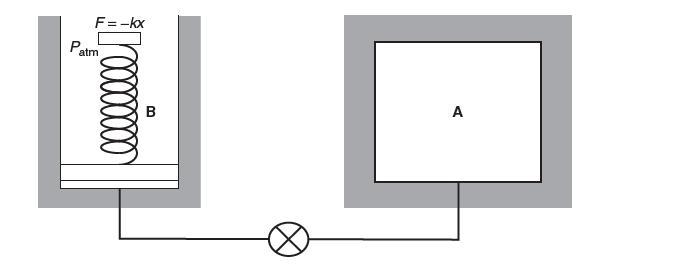
Step by Step Answer:






