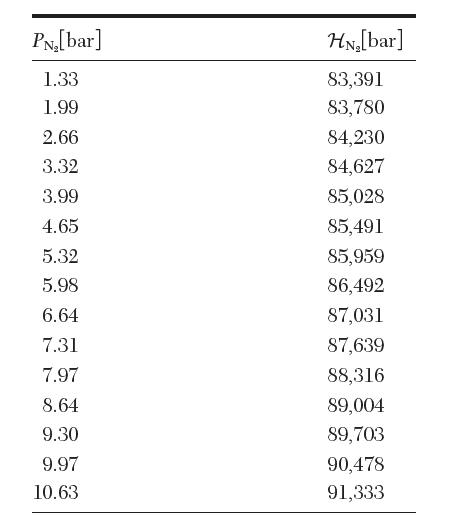
The following data are available for the Henrys law constant of N2 in H2O at 19.4C. From
Question:
The following data are available for the Henry’s law constant of N2 in H2O at 19.4°C. From these data, estimate![]()
Transcribed Image Text:
00: 8 N*
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Compute the CLV metric and then apply it in a simple breakeven analysis. The data below show the results of a Starbucks segmentation study. Each column shows the values for a typical customer in each...
-
The following data are available for 2012, regarding the inventory of two companies: Compute inventory turnover and number of days sales in inventory for both companies. Which company is handling its...
-
The following data are available for Haul-It-Away Truckers: 1. Compute the predetermined overhead rate for each of the two years, if based on (a) Direct labor hours, (b) Number of moving jobs, and...
-
Determine whether each function is one-to-one. If it is, find the inverse. f(x) = 4x - 1
-
From the following account information, prepare an income statement cost of goods sold section in proper form: Freight-In, $400; Merchandise Inventory, 12/31/17, $9,500; Purchases Discounts, $800;...
-
For a K + ?Cl ? ion pair, attractive and repulsive energies EA and ER, respectively, depend on the distance between the ions r, according to For these expressions, energies are expressed in electron...
-
Demonstrate the worksheet procedures needed to merge subsidiary accounts. AppendixLO1
-
A company has the following data: net sales, $202,500; cost of goods sold, $110,000; selling expenses, $45,000; general and administrative expense, $30,000; interest expense, $2,000; and interest...
-
Statement of LLC Liquidation Lester, Torres, and Hearst are members of Arcadia Sales, LLC, sharing income and losses in the ratio of 2:2:1, respectively. The members decide to liquidate the limited...
-
The following data are available for the Henrys law constant of O2 in benzene. From these data, estimate Ho, - ho:
-
A binary mixture of carbon dioxide and water exists in vaporliquid equilibrium at 343.15 K and 1 bar. The solubility of CO2 in the liquid has been measured as xCO2 = 0.000255. What is the Henrys law...
-
Prove that, (a) Z is a denumerable set. (b) The set Q of rational numbers is denumerable.
-
Alec is an employee who drives a 2021 Ford C-Max with a fair-market value of $32,000. He has been given the choice to have the fringe benefit reported on his W-2 either using the lease-value rule or...
-
What resource do most thinking and learning technologies rely on to be effective? a) data b) images c) gas d) robots
-
Financial Reporting Problem Marks and Spencer plc (M&S) The financial statements of M&S (GBR) are presented in Appendix A. The companys complete annual report, including the notes to the...
-
Totally Chemical is considering an investment decision project in which the organization expands into the trucking business. Totally Chemical wants to begin this investment decision project by buying...
-
Presented below is the balance sheet of Sandhill Corporation as of December 31, 2017. SANDHILL CORPORATION BALANCE SHEET DECEMBER 31, 2017 Goodwill (Note 2) Buildings (Note 1) Inventory Land Accounts...
-
How do individual markets for capital and labor relate to aggregate capital and labor markets?
-
An access route is being constructed across a field (Figure Q8). Apart from a relatively firm strip of ground alongside the field's longer side AB, the ground is generally marshy. The route can...
-
Give the structure of the major alkene formed when the hydroxide of each of the following quaternary ammonium ions is heated.
-
Outline syntheses of each of the following from aniline and any necessary organic or inorganic reagents: (a) p-Nitroaniline (c) p-Aminoacetanilide (b) 2, 4-Dinitroaniline
-
N-Nitroso amines are stabilized by electron delocalization. Write the two most stable resonance forms of N-nitrosodimethylamine, (CH3)2NNO.
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...
-
Equipment with a book value of $84,000 and an original cost of $166,000 was sold at a loss of $36,000. Paid $100,000 cash for a new truck. Sold land costing $330,000 for $415,000 cash, yielding a...

Study smarter with the SolutionInn App


