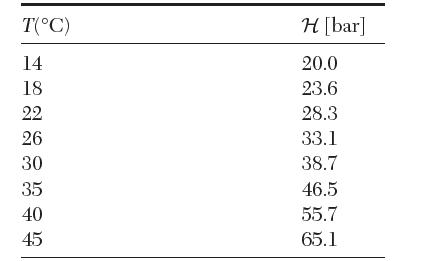
The following data are available for the Henrys law constant of O2 in benzene. From these data,
Question:
The following data are available for the Henry’s law constant of O2 in benzene. From these data, estimate![]()
Transcribed Image Text:
Ho, - ho:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Muhammad Ahtsham Shabbir
I am a professional freelance writer with more than 7 years’ experience in academic writing. I have a Bachelor`s Degree in Commerce and Master's Degree in Computer Science. I can provide my services in various subjects.
I have professional excellent skills in Microsoft ® Office packages such as Microsoft ® Word, Microsoft ® Excel, and Microsoft ® PowerPoint. Moreover, I have excellent research skills and outstanding analytical and critical thinking skills; a combination that I apply in every paper I handle.
I am conversant with the various citation styles, among them; APA, MLA, Chicago, Havard, and AMA. I also strive to deliver the best to my clients and in a timely manner.My work is always 100% original. I honestly understand the concern of plagiarism and its consequences. As such, I ensure that I check the assignment for any plagiarism before submission.
4.80+
392+ Reviews
587+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
The following data are available for 2012, regarding the inventory of two companies: Compute inventory turnover and number of days sales in inventory for both companies. Which company is handling its...
-
Compute the CLV metric and then apply it in a simple breakeven analysis. The data below show the results of a Starbucks segmentation study. Each column shows the values for a typical customer in each...
-
The following data are available for three companies at the end of their fiscal years: Required Determine the amounts indicated by question marks. Company A $ 600,000 Finished goods, January 1 Cost...
-
Graph each inequality or compound inequality. 5x - y > 6
-
From the following partial worksheet, journalize the closing entries of December 31 for A. Slow Co. A. SLOW CO. WORKSHEET FOR THE YEAR ENDED DECEMBER 31, 2016 Income Statement Balance Sheet Cr....
-
Calculate the force of attraction between a K + and an O 2- ion the centers of which are separated by a distance of 1.5 nm.
-
Demonstrate the worksheet procedures needed to eliminate the investment account. AppendixLO1
-
An ammonia solution at a high pressure is flash-vaporized at a rate of 200lbm/h. The solution contains 0.70lbm NH3/lbm, and its enthalpy relative to H2O (1, 32F) and NH3 (1, 40F) is 50 Btu/lbm....
-
The adjusted trial balance for Green Tea Company at December 31, 2021, is presented below: Account Debit Credit Cash 10,500 Accounts receivable 150,000 Prepaid rent 5,000 Inventory 25,000 Equipment...
-
Develop a computer spreadsheet or write a program to verify that the objective function in Example 8.9 gives the value A = 1399 [J/mol]. What value do you obtain for OF g E? Example 8.9 2 OF i i = (g...
-
The following data are available for the Henrys law constant of N2 in H2O at 19.4C. From these data, estimate 00: 8 N*
-
List the most important criteria when picking an option for international growth.
-
The requirement for extended disclosures for oil and gas reserves described in Chapter 2 followed a Congressional hearing on the poor disclosures that Shell Oil had for its reserves. A.Explain three...
-
Question 9 Big Data techniques implemented in the financial sector include: fraud detection O marketing email campaign O customer relationship management techniques O inventory analysis
-
Problem 8-19A Attaining notfonpmt entity variances The Redmond Management Association held its annual public relations luncheon in April Year 2. Based on the previous year's results, the organization...
-
Kay, who is not a real estate dealer, sold an apartment house to Polly during the current year (2020). The closing statement for the sale is as follows. Total selling price $190,000 Add: Polly's...
-
1 English Writing Requirement Assignment Guidelines Sem 1 2023-24 Subject code AAE1D02 Subject title Introduction to Space Exploration Credit value 3 CAR Teachers Prof. WEN Chih-Yung, Prof. WU Bo,...
-
Why is the aggregate supply curve of labor vertical?
-
Consider the activities undertaken by a medical clinic in your area. Required 1. Do you consider a job order cost accounting system appropriate for the clinic? 2. Identify as many factors as possible...
-
Which of the following amines can be prepared by the Gabriel synthesis? Which ones cannot? Write equations showing the successful applications of this method. (a) Butylamine (d) 2-Phenylethylamine...
-
Outline syntheses of each of the following arylamines from benzene: (a) o-Isopropylaniline (d) p-Chloroaniline (b) p-Isopropylaniline (e) m-Aminoacetophenone (c) 4-Isopropyl-1, 3-benzenediamine
-
Show how you could prepare each of the following amines from benzaldehyde by reductive amination: (a) Benzylamine (c) N, N-Dimethylbenzylamine (b) Dibenzylamine (d) N-Benzylpiperidine
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...
-
In the context of portfolio theory, what is diversification primarily intended to do ? A ) Increase returns. B ) Reduce risk. C ) Maximize tax efficiency. D ) Simplify investment management.

Study smarter with the SolutionInn App


